HIV & Covid-19: A Story of Concurrent Pandemics
On September 20th, Johns Hopkins’ COVID data tracker totaled the “confirmed” (note: not “official”) number of deaths from COVID-19 in the United States to surpass 675,000 – or the estimated number of deaths in the US due to the 1918-1919 H1N1 influenza pandemic (colloquially called the “Spanish flu” because Spanish media were more willing to discuss the pandemic than most other countries). Forbes, STAT, and other large news outlets ran headlines like “Covid-19 overtakes 1918 Spanish flu as deadliest disease in American history” or included statements in their articles like “It was the most deadly pandemic in U.S. history until Monday, when confirmed coronavirus deaths overtook the death toll for the Spanish Flu.”
Which, as Peter Staley pointed out, isn’t factually accurate.
Image: Twitter.com - @peterstaley (Sep 20, 2021) “Um, HIV/AIDS? 700,000 U.S. deaths (and counting), according to the http://HIV.gov https://hiv.gov/federal-response/ending-the-hiv-epidemic/overview”
Staley would quickly admit COVID-19 would or already has likely overcome the death toll of HIV in the United States. While I agree with this analysis, I would add “for now”.
The very nature of HIV has made finding a “cure” or vaccine for the virus an oft sought after “holy grail” in pharmaceutical development. While that grail may have been snatched away by the attention COVID-19 is justly generating, this isn’t the first concurrent pandemic HIV has run alongside. The Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) both refer to the H1N1 influenza outbreak of the 2009-2010 flue season a “pandemic”. The problem of course isn’t just how deadly COVID-19 is, its’ how botched the domestic and global responses have been to the disease.
Viruses, after all, are opportunistic. They have a singular purpose: reproduce. As such, viruses thrive in environments – ecosystems, if you will – that are sorely neglected, lack coordinated responses, and are largely inequitable. But we knew that. We’ve known that with regard to global and domestic health disparities data for decades. As with personal health, emerging, urgent issues in public health reduce our capacity to address existing issues effectively.
As I mentioned in previous blogs, and has been recently noted by the Global Fund, COVID-19 has drastically reduced the efficacy of existing HIV, HCV, STI, and SUD programs. Even still, Global Fund’s report proves a rather interesting point – when meeting the demands of advocates for programs to provide patients with multi-month supplies of medications, meeting people in their own neighborhoods rather than in clinics, and providing at-home testing kits, communities can be activated in care at an exceptional level. Despite the COVID-19 pandemic raging, the needs of the HIV pandemic didn’t stop. And while meeting those needs faltered some (with 4.5% fewer mothers receiving vertical transmission prevention medications, an 11% drop in prevention programming, and a 22% reduction in testing services), in some areas meeting those needs thrived. Global Fund’s report found South Africa was able to increase the number of people receiving antiretroviral therapies by more than three times the baseline, even while fighting on two fronts.
Dr. Sioban Crowley, Head of HIV at the Global Fund, pointed out these program designs are not exclusive to HIV, “If we can keep 21.9 million people on treatment, we can probably deliver them a COVID test and a vaccine.”
Indeed, with the United States’ (and the world’s) response relying heavily on expertise gained in the fight against HIV, one can reasonably ask “If we know how to beat this, why aren’t we…just doing that?”
“That” being what advocates have long asked for: a more dedicated, equitable landscape and adequate support of our public health systems. As with COVID-19, a vaccine won’t “cure” us of HIV if the rest of the world cannot access it. As with HIV, if preventative services, adequate testing, and necessary education are not readily made available to people where they are, we will continue to fail in both fights. If we don’t wish to repeat the losses we’ve already experienced in the fight against HIV, then we cannot keep making the same mistakes of kicking the costs of these investments down the road and maybe, eventually “getting to it”.
As has been said many times through the latest pandemic, “the best time to do the right thing was yesterday. The next best time to do the right thing is today.” It’s time for us to do the right thing and stop allowing backbone public health programs to fall by the wayside in the face of the next emergency. Today, for the next few years, it’s COVID. We don’t need to “wait” for that to end. There’s two pandemics occurring, it’s time we act like it.
Coverages & Pitfalls: Pandemic-Related Health Care Expansion
On September 17th, the Centers for Medicare and Medicaid Services (CMS) announced its first complete rule on health care marketplaces (federal and state – HealthCare.gov and SBEs), certain expectations of insurers participating in the health care marketplace, and a slate of other issues. This final rule serves as a regulatory tool amid a raft of information the federal government has provided regarding health insurance coverage during the pandemic.
Portions of the rule were fairly well expected (extending the annual open enrollment period from the slimmed down 45 days the previous administration imposed and revamping the “navigators” program), while other portions sought to more narrowly address – read “stop” or “reverse” – changes from the previous administration (specifically those introduced in 2018 and others introduced on January 19, 2021). The rule also aims to address some pressing concerns from legislators and health care access advocates about affordability of insurance as the economic future of the country remains unstable with the COVID-19 pandemic still wreaking havoc on much of the country.
One September 14th and 15th, the Census Bureau and the Department of Health and Human Services (HHS), respectively, issued data related to insurance coverage among residents of the United States in 2020 and 2021. The Census data diverged slightly from the HHS data in that the Census data did not show an increase in the number of people enrolled in Medicaid from 2018 to 2020, whereas previously released CMS data had shown a substantial increase (15.6% or about 10.5 million people) in Medicaid and Children’s Health Insurance Program (CHIP) enrollment from February 2020 through March 2021. A report from the Urban Institute cites potential for the remainder of 2021 to net an additional 17 million people enrolled into the safety net health insurance programs. All of this coincides with the Census data showing the uninsured rate was near static from 2018 to 2020 (8.6% compared to 8.5%).
This is a pretty remarkable comparison, given the pandemic’s effects on the country’s economy. The Census does cite a reported drop in employment related coverage during 2020, with the highest rate of employer sponsored coverage drop occurring among those employed less than full-time. Indeed, the data shows the largest drop in employer sponsored coverage occurred for those at the lowest end of the compensation scale.
Part of why tying together the employment data of the Census and the Medicaid and CHIP enrollment growth data together is to better understand the severe risks to vulnerable people and families as the seeks to wrap up the public health emergency declarations (likely sometime next year). While the administration’s payment rule highlights efforts to keep afloat lower- and middle-income families afloat and insured, the same can’t be said of the lowest-income earners. While the Biden Administration has extended the period for states to return to enrollment recertifications for Medicaid, federal matching funds (a boosted benefit to states during the pandemic) are expected to end on a similar timeframe, giving states some added financial motivation to move through disenrolling current recipients quickly, rather than in a staggered fashion.
While we outlined steps state Medicaid programs and safety net providers could take at the wrap up of the PHE in a blog earlier this month, the Biden Administration must seek even more moves than currently planned, to ensure “back to normal” doesn’t amount to “back to broke” for low-income families across the country.
Veterans Linkage to Care: Perspectives on HIV, Viral Hepatitis, Opioids & Mental Health
Approximately 8 percent of the U.S. population are Veterans, numbering over 18 million Americans with most of them being males and older than nonveterans. But those demographics will change in the coming years, with significant increases in ranks among women and minorities. As a society, we tend to view these men and women formerly in uniform as larger than life figures capable of overcoming almost any odds. The reality, however, is there are numerous ongoing public health challenges faced by Veterans in this country once discharged from the military – among them HIV, Hepatitis C, opioid dependence, and mental health conditions. As a society, don’t we owe it to them to provide the most timely, appropriate linkages to care and treatment?
In 2019, there were 31,000 Veterans living with HIV seeking care within the Veterans Affairs (VA) healthcare system. Additionally, 3.4 million Veterans were eligible for HIV screening. Navigating the VA is challenging enough for our Veterans, but imagine doing so after first being diagnosed with a lifelong, chronic illness like HIV/AIDS? Although no longer a death sentence, Veterans need to learn how to steer living with HIV in what seems like a battlefield of complex bureaucratic systems, simply to start their care and treatment. For Veterans, staying connected to appropriate levels of care continues to be vital for many reasons.
For example, pulmonary hypertension – a blood pressure condition that affects the lungs and heart – is higher among Veterans living with HIV than in veterans who don’t have an HIV-positive diagnosis. What adds an extra level of concern is that Veterans with a CD4 count below 200 are also at higher risk of pulmonary hypertension, including Veterans who have viral loads higher than 500 copies per mL. Pulmonary hypertension within itself is a rare condition but that is exactly the reason why Veterans needs to remain linked to their healthcare providers. Some healthcare providers may not be actively probing for rare conditions like pulmonary hypertension and thus the condition and its possible progression will go largely undiagnosed. This further places into perspective the wide net needed in appropriate, timely HIV care and treatment that goes beyond taking antiretroviral (ARV) medication to achieve viral suppression.
Advances in HIV medicine – namely the introduction of the highly active antiretroviral treatment in 1996 – changed how people can live their lives after an HIV-diagnosis. Whereas people living with HIV who are virally suppressed have the same life expectancy as their non-positive counterparts, they’re also prone to age-related conditions and other co-morbidities, such as the previously discussed pulmonary hypertension. What this also means is that living longer, fuller lives also opens-up the door to emotional distresses.
Newly enlisted service members cycle through intense emotions when shifting from civilian life to the demands of military culture. Post discharge, Veterans can find themselves yet again cycling through acute reactions as they struggle to respond back into the reintegration of the everyday family and civilian life. As a result, studies have shown that incidences of ischemic stroke, the most common type, is more prevalent in Veterans who are HIV-positive dually diagnosed with depression in comparison to Veterans who don’t have a positive diagnosis without depression. This is significant because a common psychological effect of depression is isolation. Without proper linkages to care, so many human pathways of connectivity can begin to become severed. Positive behaviors and patterns begin to change, and this is a dangerous mental state to be in not only as a Veteran struggling with civilian life, but also maintaining the healthy and consistent level of care and treatment that is needed for Veterans living with HIV. It opens the door to poor medication adherence, decreased social networks, and increased likelihood of substance use disorder. These landmines are crucial markers to ensure Veterans living with HIV are kept engaged in their treatment plans. Likewise, all clinicians need to do the same by remembering to evolve with their clients to continue providing them with the services that they need and deserve.
Another silent threat facing both Veterans and nonveterans alike is Hepatitis C (HCV). The Centers for Disease Control & Prevention (CDC) estimates there are nearly 2.4 Americans living with HCV. It continues to remain a public threat to the general population, but it particularly relevant to address how the silent epidemic is impacting Veterans.
If left untreated, HCV can be fatal because it can lead to cirrhosis of the liver. Veterans experience chronic HCV at three times the rate of the general population, with 174,000 Veterans in active care within the VA system. So, what factors need to be considered by Veterans seeking testing and treatment options for HCV? After all, modern medicine continuously changes the landscape of the available medical treatment options, and the constant reevaluation can be overwhelming. Fortunately, newer HCV therapies have made it a little easier. A qualitative analysis of 29 Veterans who were looking into HCV treatment, 35 total factors were of interest were identified. Of this set of 35 attributes, the top reported were treatment efficacy, physical side effects, new antiviral drugs in the pipeline, liver condition, and psychological side effects.
While the report’s findings aren’t necessarily surprising, how they structured their analysis is important. The Veterans in this study were placed in one of three categories that identified their personal stage of change – which were contemplating treatment, recently declined treatment, and recently initiated treatment. Successful linkages to care involve acknowledging where clients are in the process because it helps to identify and structure a patient centered treatment plan. What is important to remember is that each stage of change is shaped by the personal lived experiences clients are currently experiencing. Some of these subfactors are important social systems that they interchangeably occupy like family, friends, work, religion, and perhaps other various community engagements. All of which can greatly affect the decision-making process when considering treatment. Clinicians across the board need to have a clear picture as to what their client’s value and integrate those value systems into the appropriate levels of care to maximize the effectiveness of their treatment.
This same study uncovered another point of interest that is worth mentioning. When it came to gender, 50% of women compared to 14% of men, reported having concerns with social attributes like stress on partnerships and stigma associated with a disease. Additionally, women also reported concerns about maintaining their privacy within the systems that they occupy. In some ways these results are not surprising given the long history of women being undervalued and overexposed within society. That said, what this does highlight is how the concept and execution of healthcare needs the integration of a vast interpersonal team across a diverse and all-encompassing platform that has the capability to target these pockets of influence.
Healthcare disparities, unfortunately, exist across a wide spectrum within our medical framework and the VA isn’t immune from it. For minority Veterans with hepatitis C, seeking treatment are faced with unique barriers. For example, an HCV-diagnosis is four times more likely among minority Veterans compared to the general population. The VA’s Office of Health Equity (OHE) has done some great work in eliminating health disparities among minority Veterans with HCV, including testing. Testing is made available to all Veterans who are enrolled in the VA; they have treated more than 123,000 Veterans, and successfully cured more than 105,000 Veterans. The VA’s vigorous approach to its mission has been met with great results as race and ethnicity proportions are being treated equally with no population higher than the other. Effective strategies like video telehealth, the use of nonphysician providers, and electronic data tools for timely patient tracking and outreach have allowed the VA to expand their services to better address gaps in care. Work like this is needed across VA systems and local communities to minimize the gaps that are all too often seen in minority groups especially when there are 50,000 Veterans who are undiagnosed for HCV.
Any discussion about linkages to care needs to address the risk association between Hepatitis C and opioids. Since 2010, there have been correlating spikes in both. According to the CDC, HCV cases have nearly tripled between 2010-2015, and during this time the growing use of opioids exploded thanks to OxyContin, Vicodin, morphine, and fentanyl.
Like the general population, substance use disorder can be an inherited experience for Veterans, sometimes exacerbated by the effects of military culture. As a result, 1.3 million Veterans experience levels of substance use disorder. A study by the VA Health System in 2011 indicated that Veterans, when compared to the general population, are twice as likely to experience death from an opioid overdose incident. The biggest leading factor in this is prescription opioid medication and it continues to increase. In 2005, 4 percent of service members reported misusing their prescription medication. Three years later, 11 percent of service members reported the same misuse. The challenge here is that military culture demands a high level of sacrifice, which often comes with potential risk factors like bodily injuries and exposure to traumatic events. Both can be a slippery slope. Physical injury begins to be a major factor almost immediately after enlisting. Service members are pushed daily to exercise and ushered through a series of combat drills that will no doubt include heavy equipment. The body has a great ability to adapt and strengthen itself but like anything else, it has its limits. If this sets the stage for a revolving door of service members in physical pain, the natural course of action would be to provide medication to offset these symptoms. And just like that, accessibility without effective evaluations become the gateway to opioid substance use.
In the same fashion, traumatic events can leave service members feeling disconnected from where they’d like to be both emotionally and physically. In military culture, perception of strength is reality and as such, seeking services for mental health is often challenging for servicemembers as they don’t want to appear weak, so they suffer in silence. But that is exactly the reason why work is needed to change this outcome. Military culture to a very large degree is unwavering. It needs to build soldiers and do that; it needs to condition enlistees. However, it would be beneficial if clinicians and doctors within military culture to incorporate better systems of evaluation when it comes to pain management. This would also need to extend into the various VA systems that service members have access to. Relationships and bonds are obviously built within military culture and their importance may be of great benefit when combating the negative effects of stigma associated with mental health trauma. Community programs can be fostered and guided by various ranking officers to establish a sub community where conversations of real-life experiences demonstrate that a soldier of any rank can be supported by the comrades and communities that they protect.
But accessibility is a two-way street. Clients should have the ability to gain access to healthcare to receive treatment for various medical concerns. Clinicians or outreach programs should be able to have access to community members that need a particular public health service. Syringe services programs (SSP) introduced in the 1980s, have been adopted by the VA system to reduce the harm for Veterans who inject drugs . Veterans who utilize SSP’s can receive substance use and mental health services with the VA including additional services through an SSP program like vaccinations and naloxone, which helps to prevent an accidental overdose. Veterans benefit from community-based programs like this even with the controversies that the program may still carry since its inception. This program has been proven effective in reducing transmission of disease like HIV and Hepatitis C. While this program isn’t stopping the use intravenous drug use, it does open the door for Veterans who may be in a place mentally to accept help. Programs like this are a great hub to access community members and have conversations about recovery services. Like most things in life, addiction is complex involving a multitude of factors that contribute to the addictive behavior. Drugs are the symptom, but the person is the real key to the solution within the equation. Lived experiences matter when looking at public health issues across the board. How people experience live greatly shapes how they decide to show up for it, especially in challenging times. If there are 343,000 Veterans who use illicit drugs, then effective and targeted programs need to be in place not only at VA systems but also in their surrounding communities. One of the great aspects of SSP programs is that it targets Veterans by how they are currently living with a substance use disorder, and while strengthening community engagement through public service.
Military culture and trauma are often associated with one another, but it isn’t always linked to deployment. That said, combat-related post-traumatic stress disorder (PTSD) is quite prevalent among active-duty service members, as well as Veterans. For service members nearing active-duty discharge, a diagnosis of PTSD may change the status of their discharge, greatly affecting the outcome of receiving services from the VA. The term “bad papers” is used within military culture to signify that a Veteran has been discharged unfavorably. A status discharge of other than-honorable is essentially the kiss of death because it means that a Veteran will not be able to access services through the VA. What is interesting about this status is that it is given for felonies, those absent without official leave (AWOL), desertion and Veterans with drug offenses. The issue then becomes the consequent behaviors of Veterans struggling with PTSD who turn to substance use to cope and who then also begin to have behavioral changes which affects level of performance on all fronts. Veterans carry an immense sense of pride for their service, and rightfully so. They have stepped in roles that most people don’t have the courage to do so. As an evolving clinician, seeing a Veteran struggle with PTSD due to the natural climate of what their duty demands of them, and then being shut out of benefits that are crucial to their mental health is just unacceptable. Discharge review boards really need to reconsider the criteria for evaluating Veterans who suffer from traumatic events. Not doing so sends a message that devalues the sacrifices that they have made which then perpetuates the stigma associated with their discharge status, but also reinforces the negative outlook of mental health within military culture. Veterans should not have to suffer in silence for enduring what was demanded of them and then be casted aside because their organization feels that their value has expired.
In 2016, over 1.5 million of the 5.5 million Veterans who entered the VA hospitals, had PTSD or other mental health diagnosis. That’s a staggering number especially when you consider the constant influx of Veterans who are returning home from deployment. Compared to the general population, suicide death rates are higher in Veterans, and furthermore female Veterans have a suicide rate of 35 per 100,000. Mental health services within the VA system have been on ongoing challenge as they try to meet the demand that Veterans need for crisis-intervention. As it is, mental health services are expensive for nonveterans, and even those who are insured may not have the adequate coverage to seek mental health services during a crisis episode. For Veterans returning home experiencing a mental health condition, this is disastrous as communities and VA systems both struggle to provide crisis stabilization and interventions. As a result, many Veterans experience depression on top of another mental health diagnosis like PTSD. Homelessness in Veterans is also increasing with more than 107,000 Veterans who are displaced. All of this is a perfect storm for a Veteran to feel like all hope is lost and consider suicide and reports reflect that with 21 Veterans, on average, dying of suicide every day. In society, there is a lot of talk about how all human beings are deserving of human equity. Human equity should include the ability to access mental health services (and healthcare as a whole), and the capability to navigate healthcare systems by having the support of organizations, communities, and effective public policy.
The military culture’s sphere of influence is completely different from civilian life. It is a complex system demanding everything military personnel can give, but it can often fall short when the time comes to giving back to Veterans. The sad truth is, Veterans often confront too many barriers when attempting to access appropriate timely care and treatment. It isn’t a secret that mental health disorders and other numerous challenges, such as substance use disorder, stem from military service-related experiences. Yet, systems in place for Veterans are inadequately structured to meet the numerous public health issues confronting Veterans and, subsequently, their families. Accessibility to healthcare services, including mental health, needs to encompass a wide net of effective policies and programs but also infused with the knowledge of how Veterans occupy the various systems that they live in and are affected by them. Too often in healthcare, clients are evaluated solely based off a diagnosis and without ever including who they are and their lived experiences. These are large, missed opportunities for clinicians to home in on invaluable information that can help formulate more effective treatment plans in conjunction with innovative and effective public policy. Hubs like VA systems are a great resource for Veterans, but we need to make sure the avenues of accessibility remain open for all Veterans that are eligible. It is very rare that a solution to a problem ever stands alone, and this perspective should continue to be a driver as community engagement and expansion in healthcare accessibility is needed. Veterans answered the call of duty without hesitation so now we must not drag our feet when Veterans need us the most in a war that poor public policy, lack of community programs and military culture has waged on them.
References:
Belperio, A,. Korshak,L., & Moy, E. (2020). Hepatitis C Treatment in Minority Veterans. Office of Health Equity. Retrieved online at https://www.va.gov/HEALTHEQUITY/Hepatitis_C_Treatment_in_Minority_Veterans.asp
Burek, Gregory, M.D. (2018). Military Culture: Working With Veterans. The American Journal of Psychiatry Residents’ Journal. Retrieved online at https://ajp.psychiatryonline.org/doi/pdf/10.1176/appi.ajp-rj.2018.130902
Centers for Disease Control & Prevention (2018). CDC Estimates Nearly 2.4 Million Americans Living with Hepatitis C. U.S. Department of Health & Human Services. Retrieved online at https://www.cdc.gov/nchhstp/newsroom/2018/hepatitis-c-prevalence-estimates-press-release.html
Duncan, M. S., Alcorn, C. W., Freiberg, M. S., So-Armah, K., Patterson, O. V., DuVall, S. L., ... & Brittain, E. L. (2021). Association between HIV and incident pulmonary hypertension in US Veterans: a retrospective cohort study. The Lancet Healthy Longevity.
HepMag (2019). Veterans and Hepatitis C. Retrieved online at https://www.hepmag.com/basics/hepatitis-c-basics/veterans
Hester, R. D. (2017). Lack of access to mental health services contributing to the high suicide rates among veterans. International journal of mental health systems, 11(1), 1-4.
Maguire, Elizabeth (2021). Providing clean syringes to Veterans who inject drugs. VAntage Point (Blog). Retrieved online at https://blogs.va.gov/VAntage/89943/providing-veterans-inject-drugs-clean-syringes/
Military Officers Association of America blog (2017). Veterans and Opioid Addiction. Retrieved online at https://www.moaa.org/content/publications-and-media/features-and-columns/health-features/veterans-and-opioid-addiction/#.YNxcrmTZy_0.twitter
Pebody.,R. (2018). Life expectancy for people living with HIV. AIDSmap. Retrieved online at https://www.aidsmap.com/about-hiv/life-expectancy-people-living-hiv
Schultz, Jennifer (2017, November 10). Veterans By the Numbers. The NCSL Blog. Retrieved online at https://www.ncsl.org/blog/2017/11/10/veterans-by-the-numbers.aspx#:~:text=There%20are%2018.8%20million%20veterans%20living%20in%20the,rise.%20Veterans%20tend%20to%20be%20older%20than%20nonveterans
Sico, J. J., Kundu, S., So‐Armah, K., Gupta, S. K., Chang, C. C. H., Butt, A. A., ... & Stewart, J. C. (2021). Depression as a Risk Factor for Incident Ischemic Stroke Among HIV‐Positive Veterans in the Veterans Aging Cohort Study. Journal of the American Heart Association, 10(13), e017637.
Sisk, R. (2021). ‘Dirty, Embarrassing Secret:’ Veterans with PTSD Struggle to Shed Stigma of Bad Paper Discharges. Military. Military.com. Retrieved online at https://www.military.com/daily-news/2021/04/21/dirty-embarrassing-secret-veterans-ptsd-struggle-shed-stigma-of-bad-paper-discharges.html
Substance Abuse and Mental Health Services Administration. (2020). 2019 National Survey on Drug Use and Health: Veteran Adults. Retrieved online at https://www.samhsa.gov/data/sites/default/files/reports/rpt31103/2019NSDUH-Veteran/Veterans%202019%20NSDUH.pdf
U.S Department of Veterans Affairs, (2020). Human Immunodeficiency Virus (HIV) - VA IS THE LARGEST SINGLE PROVIDER OF HIV CARE IN THE UNITED STATES. Fact sheet. Retrieved online at https://www.hiv.va.gov/pdf/HIV-program-factsheet.pdf
Wisely, Rene (2018). Why Are Hep C Infections Skyrocketing? Opioid Abuse to Blame. Michigan Medicine. Retrieved online at https://healthblog.uofmhealth.org/hep-c-infection-and-drug-abuse
Zuchowski, J. L., Hamilton, A. B., Pyne, J. M., Clark, J. A., Naik, A. D., Smith, D. L., & Kanwal, F. (2015). Qualitative analysis of patient-centered decision attributes associated with initiating hepatitis C treatment. BMC gastroenterology, 15(1), 1-10.
Biden Administration’s Healthcare Future is One of Promise & Peril
Last month, the Biden Administration issued a press release outlining a look toward the future of American health care policy. Priorities in the presser include ever elusive efforts around prescription drug pricing and items with steep price tags like expanding Medicare coverage to include dental, hearing, and vision benefits, a federal Medicaid look-alike program to fill the coverage gaps in non-expansion states, and extending Affordable Care Act (ACA) subsidies enhancements instituted under the American Rescue Plan (ARP) in March. Many of these efforts are tied to the upcoming $3.5 trillion reconciliation package.
President Biden renewed his call in support of the Democrats effort to negotiate Medicare prescription drug costs, enshrined in H.R. 3. Drug pricing reform has been an exceptional challenge despite relatively popular support among the voting public, in particular among seniors. The pharmseutical industry has long touted drug prices set by manufacturers do not represent the largest barriers to care and mandating lower drug costs would harm innovation and development of new products. Indeed, for most Americans, some form of insurance payer, public or private, is the arbiter of end-user costs by way of cost-sharing (co-pays and co-insurance payments). To even get to that point, consumers need to be able to afford monthly premiums which can range from no-cost to the enrollee to hundreds of dollars for those without access to Medicaid or federal subsidies. The argument from the drug-making industry giants is for Congress to focus efforts that more directly impact consumers’ own costs, not health care industry’s costs. Pharmaceutical manufacturers further argue mandated price negotiation proposals would harm the industry’s ability to invest the development of new products. To this end, the Congressional Budget Office (CBO) recently released a report giving some credence to this claim. The CBO’s report found immediate drug development would hardly be impacted as those medications currently “in the pipeline” would largely be safe, but a near 10% reduction in new drugs over the next 30 years. While new drug development has largely been focused on “personalized” medicine – or more specific treatments for things like cancer – implementing mRNA technology into vaccines is indeed a matter of innovation (having moved from theoretical to shots-in-arms less than a year ago). With a pandemic still bearing down on the globe, linking the need between development and combating future public health threats should be anticipated.
The administration’s effort to leverage Medicare isn’t limited to drug pricing. Another tectonic plate-sized move would seek to expand “basic” Medicare to include dental, hearing, and vision coverage. Congressional Democrats, while generally open to the idea, are already struggling with timing of such an expansion, angering Senator Bernie Sanders (I-VT) by suggesting a delay until 2028. While any patient with any ailments related to their oral health, hearing, and vision will readily tell you these are critical and necessary coverages, even some of the most common of needs, the private health care insurance industry generally requires adult consumers to get these benefits as add-ons and the annual benefit cap is dangerously low (with dental coverage rarely offering more than $500 in benefit and vision coverage capping at one set of frames, both with networks so narrow as to be near meaningless for patients with transportation challenges). While the ACA expanded a mandatory coverage for children to include dental and vision benefits in-line with private adult coverage caps, the legislation did nothing to mandate similar coverages for adults and did not require private payers to make access to these types of care more meaningful (expanded networks and larger program benefits to more accurately match costs of respective care).
The other two massive proposals the Biden Administration is seeking support for, more directly impact American health care consumers than any other effort from the administration: maintaining expanded marketplace subsidies and a federal look-a-like for people living in the 12 states that have not yet expanded Medicaid under the ACA’s Medicaid expansion provisions. The administration has decent data to back this idea, as the Centers for Disease Control and Prevention released a report showing a drop in the uninsured rate from 2019 to 2020 by 1.9 million people, largely attributed by pandemic-oriented programs requiring states to maintain their Medicaid rolls. The administration and Congressional Democrats are expected to argue subsequently passed legislation allowing for expanded subsidies and maintained Medicaid rolls improved access to and affordability of care for vulnerable Americans during the pandemic. As the nation rides through another surge of illness, hospitalizations, and death from the same pandemic “now isn’t the time to stop”, or some argument along those lines, will likely be the rhetoric driving these initiatives.
Speaking of the pandemic, President Biden outlined his administration’s next steps in combating COVID-19 on Thursday, September 9th. The six-pronged approach, entitled “Path out of the Pandemic”, includes leveraging funding to support mitigation measures in schools (including back-filling salaries for those affected by anti-mask mandates and improving urging the Food and Drug Administration [FDA] to authorize vaccines for children under the age of 12), directing the Occupational Safety and Health Administration (OSHA) to issue a rule mandating vaccines or routinized testing for employers with more than 100 employees (affecting about 80 million employees) and mandating federally funded health care provider entities to require vaccination of all staff, pushing for booster shots despite the World Health Organization’s call for a moratorium until greater global equity in access can be attained, supporting small businesses through previously used loan schemes, and an effort to expand qualified health care personnel to distribute COVID-19 related care amid a surge threatening the nation’s hospitals ability to provide even basic care. Notably missing from this proposal are infrastructure supports for schools to improve ventilation, individual financial support (extension of pandemic unemployment programs or another round of direct stimulus payments), longer-term disability systems to support “long-COVID” patients and any yet-unknown post-viral syndromes, and housing support – which is desperately needed as the administration’s eviction moratorium has fallen victim to ideological legal fights, states having been slow to distribute rental assistance funds, and landlords are reportedly refusing rental assistance dollars in favor of eviction. While the plan outlines specific “economic recovery”, a great deal is left to be desired to ensure families and individuals succeed in the ongoing pandemic. Focusing on business success has thus far proven a limited benefit to families and more needs to be done to directly benefit patients and families navigating an uncertain future.
President Biden did not address global vaccine equity in his speech, later saying a plan would come “later”. The problem, of course, is in a viral pandemic, variant development has furthered risks to wealthy countries with robust vaccine access and threatened the economic future of the globe.
To top off all of this policy-making news, Judge Reed O’Connor is taking another swing at dismantling some of the most popular provisions of the ACA. Well, rather, yet another plaintiff has come to the sympathetic judge’s court in an effort to gut the legislation’s preventative care provisions by both “morality” and “process” arguments in Kelley v. Becerra. The suit takes exception to a requirement that insurers must cover particular preventative care as prescribed by three entities within the government (the Health Resources Services Administration – HRSA, the Advisory Committee on Immunization Practices – ACIP, and the Preventative Services Takes Force – PSTF), which require coverage of contraceptives and pre-exposure prophylaxis (PrEP) with no-cost sharing to the patient, among a myriad of other things – including certain vaccine coverage. By now, between O’Connor’s rabid disregard for the rights of lesbian, gay, bisexual, and transgender Americans and obsessive effort to dismantle the ACA at every chance he can – both to his own humiliation after the Supreme Court finally go their hands on his rulings – Reed O’Connor may finally have his moment to claim a victory – I mean – the plaintiffs in Kelley may well succeed due to the Supreme Court’s most recent makeover.
As elected officials are gearing up for their midterm campaigns, how these next few months play out will be pretty critical in setting the frame for public policy “successes” and “failures”. Journalists would do well to tap into the expertise of patient advocates in contextualizing the real-world application of these policies, both during and after budget-making lights the path to our future – for better or worse.
A Different Booster: HBV Vaccines among PLWHA
Because of the shared transmission vectors between HIV and Hepatitis B (HBV), the rate of co-infection is about 10% in the United States, according to the Centers for Disease Control and Prevention (CDC). As a result, people living with HIV (PLWHA) are more likely to experience adverse health impacts including cirrhosis and certain types of liver cancers. A small study conducted in Chile took a look at the recommended HBV vaccine schedule among adults living with HIV and HBV antibody uptake and potentially finding cause for a “high dose” fourth shot to be added into the series for PLWHA.
A giant asterisk belongs on the study’s findings, labeled “deserves further study consideration”. Despite being double-blinded, the study’s greatest weakness included participant pool size (right around 100 participants) and clinical selection criteria (which remains an issue in clinical trial work, generally speaking). In order to be considered for the study, participants generally had to present with an undetectable HIV viral load and no other comorbidities, ruling out application of the resulting data to most PLWHA and especially most long-term survivors or people experiencing barriers to care or medication adherence concerns – or those most likely to be impacted by HIV and HBV co-infection.
The study sought to examine the need for revaccination among PLWHA. Of note, the CDC’s “Pink Book” on HBV does not recommend “boosters” unless a particular “low” threshold of HBV antibodies is met, nor does the publication recommend for routinized serological testing among people who have previously received a vaccine. Therein lies a program and policy problem. We’ll get to that in a moment.
As a result of selection bias favoring those with more ideal circumstances, few participants dropped out of the trial. The study itself found that a fourth and “stronger” dose of vaccine improved antibody responses among people with “well controlled” HIV with an improved HBV antibody response from 50.9% in the low-dose arm of the study to 72% among the high-dose arm of the trial. After a one-year follow up, 80% of participants of the high-dose arm still had sufficient antibody titers, whereas only 39% of the standard-dose arm still had sufficient antibodies for protection.
While Ryan White and CDC funded clinical care programs for PLWHA require HBV monitoring and vaccination efforts as part of their grant funding, few entities necessarily do and almost no private providers do. Federally-funded providers may screen upon intake or initial labs but maintenance screening is not a priority in terms of clinical data collected on a given patient. Even on-site audits from these funders can sometimes look like reviewing particular case files and discussing details but the HBV conversation is not pressing. Rather, a review of intake data can suffice depending on the clinical auditor/consultant (site-visits and audits are often conducted under the supervision of the funding agency but only actually audited by consultants, including staff from other funded clinics).
Public funders aiming to end HBV and the unjust circumstances in which PLWHA are not educated by their providers on the other risks to their health should shift some focus to emphasize the need for preventative care – especially vaccines. Provider education for these publicly funded clinics should include the need to routinize HBV antibody monitoring not just as a concern on behavioral risk factors continuing in a client’s life but because HBV immunity is clearly not necessarily a given, regardless of prior vaccination history.
While the study suggests the need for investigating further, with regard to efficacy of HBV vaccines among PLWHA, the larger question - given the nation-wide rush for another vaccine (and boosters) - creating more robust standards of care among a population known to have immunological “memory-loss” due to the particular cells “attacked” by HIV seems to be in order. Part and parcel to that is tying this level of necessary education to funding and licensure could improve the quality of care PLWHA receive, especially those of low-income and otherwise marginalized identities.
Amid HIV Outbreaks, Covid-19 Fractures Existing Public Health Efforts
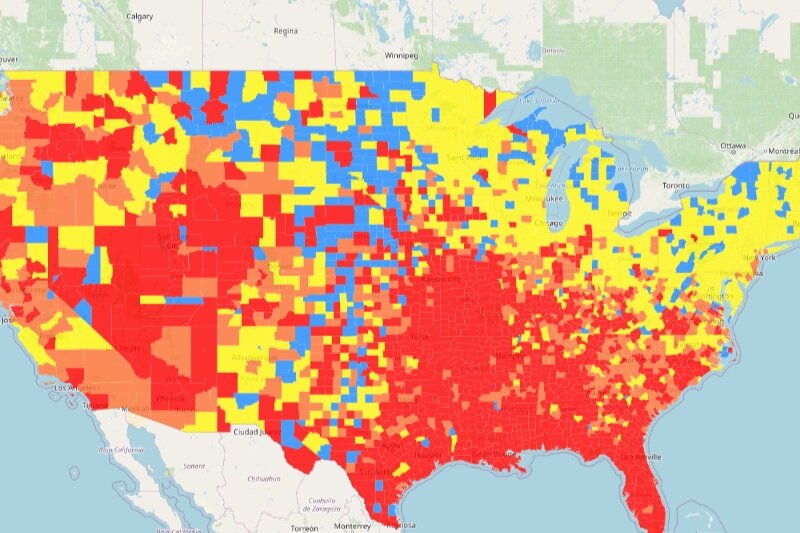
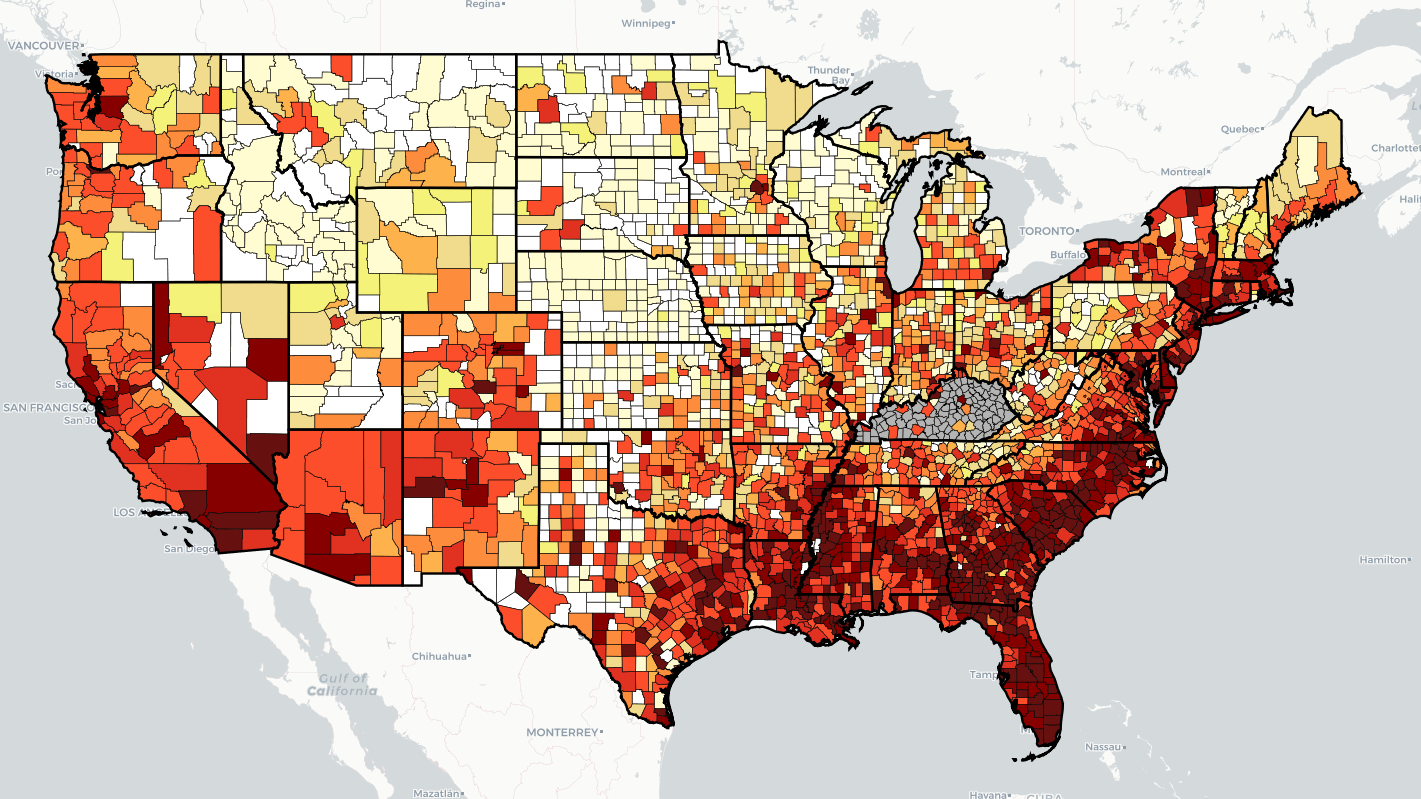
“Every health disparity map in the United States is the same,” Alex Vance, Senior Director of Advocacy and Public Policy at the International Association of Providers in AIDS Care, has repeatedly stated when discussing the COVID-19 pandemic and the very real risks of “back sliding” in our moderate advancements in the United States’ effort to End the HIV Epidemic. There’s no better way to demonstrate this than by showing you.
As another “wave” of Covid-19 ravages the country, increasing cases, hospitalization rates, and eventually deaths, existing public health needs around HIV, viral hepatitis, and sexually transmitted infections continue to be strained, pushed to the back burner and most often in geographic areas where we need to be able to juggle more, not less; particularly across the South. Unlike Covid-19 outbreaks, which receive fairly immediate attention in terms of reporting and response, HIV outbreaks can and do often take a year or more to notice and begin action to address. While there’s concern about juggling the demands of addressing concurrent pandemics, some (certainly not all) of this reporting and response is beginning to speed up with regard to HIV.
West Virginia’s Kanawha County HIV outbreak is considered a 2020 outbreak, though just recently received a report from the Center’s for Disease Control & Prevention (CDC) on next steps and recommendations to address. Despite these recommendations including supporting syringe exchange programs and community-based services, local officials continue a politically oriented response by seeking to limit the support of syringe exchange programs, threatening their ability to operate and aid in addressing the needs of the local community. Another outbreak in Michigan’s Upper Peninsula was identified in July of this year, with rural Kalkaska County reporting a higher rate of new HIV diagnoses than even Detroit. Kalkaska County is so similarly situated to Scott County, Indiana, it’s striking. Louisville, Kentucky has reported almost as many new HIV diagnoses in the first half of 2021 as the area does annually. And Duluth, outside of Saint Louis, Minnesota, joined neighboring Hennepin and Ramsey Counties with declared outbreaks tied to 2019 and 2018, respectively.
What’s important to note about 2020’s marked reduction in HIV screenings is not just the delay in newly diagnosing people living with HIV, but the lack of ability to link people to care upon diagnosis, beginning antiretroviral therapy and taking steps to reduce the person’s viral load. With at-home testing being the substitute offering, linkage to care and counseling for people self-testing may be hampered according to some concerned advocates. Achieving viral suppression also reduces the possibility of transmitting HIV by way of sexual contact to zero (“Undetectable = Untransmittable”) – creating a process where people living with HIV are not just a patient group needing identification, but play a critical role in preventing new transmissions.
In reviewing the possibilities of delayed care and delayed screening, public health officials and advocates should remember a new diagnosis is not necessarily indicative of a new transmission. A potential problem in and of itself, in assessing Covid-19 disruptions in screening and care, is the possibility of a “bottle neck” of new testing revealing new HIV diagnoses which otherwise might have been identified in the previous year if not for stay-at-home orders, education and public awareness campaigns, and community health care providers having had to take a step or shift gears entirely from HIV to Covid-19. It will take years for us to truly understand the breadth and reach of Covid-19 on the world’s only concurrent pandemic, even in the “most advanced country in the world”.
What can already be well-appreciated and should be well-understood is we cannot afford to keep asking community-based health care providers and community partners in combatting the domestic HIV epidemic to keep sacrificing HIV screening and linkage to care in order to address Covid-19. What must be prioritized is funding, programming, training, and most importantly hiring of new talent in addition to existing programs in order to address both sets of needs.
As I summarized in a previous blog, capacity has been breached, we cannot afford to keep asking public health to do more with less and expect to succeed in addressing Covid-19, HIV, viral hepatitis, STIs, or the opioid epidemic.
Post-PHE: Continuity in Care for Vulnerable Populations is Critical
On July 20th, the United States extended its existing declaration of Public Health Emergency (PHE) in response to the COVID-19 pandemic for 90 days. Previously, the PHE had been renewed 6 times under the previous and current administrations. The PHE declaration may be extended past October 20th, 2021, should the Secretary of Health and Human Services (HHS), Xavier Becerra, renew the declaration.
Pandemic response and relief funding from the federal government has come with strings attached in order to ensure those funds are directed toward those who need the help the most. Most of these strings operate as both “stick and carrot” and one of the more interesting “carrots” was the increase of federal dollars supporting state Medicaid programs for the trade-off of maintaining those Medicaid rolls, temporarily ceasing redetermination and reenrollment activities, allowing people to remain on Medicaid rolls through the PHE without having to go through the usual hoops of proving their eligibility on a more regular basis.
While the previous administration directed states to anticipate a return to usual work after the PHE, engaging in a massive redetermination effort inside of 6 months of the PHE ending, earlier this month, the Biden administration informed states that redetermination period would be extended to 12 months in order to avoid an artificial “bulge” of redeterminations and eligibility checks and, ultimately, a potential annual cycle of concentrated renewals in a short window of time. It’s important to remember, as we discuss Medicaid redetermination, rules vary by state and those disenrolled during redetermination are not necessarily ineligible, they may merely not have had an opportunity to respond to a request for information for a variety of reasons.
The guidance from the Biden Administration speaks directly to this issue, stating states should consider providing a “reasonable” amount of time for clients to provide additional information for redetermination. The administration’s idea of a reasonable amount of time is 30 days. Louisiana, as an example, typically only allows for 10 days from the date in which a paper letter has been mailed to a Medicaid recipient for that same recipient to respond. If the recipient is ill, needs to gather supporting evidence from multiple sources, the mail is slow, or any number of factors outside of their control, they may be unceremoniously disenrolled. A mass redetermination effort in a shortened period of time runs a significant risk of disenrolling otherwise eligible clients but for a process that leaves less than no room for delay or mistake. Indeed, a 2019 report from Louisiana’s Health department found that 85% of eligibility cases were closed for a lack of response to a request for information. Louisiana isn’t alone in these burdensome processes, which on the surface, appear to be aimed at discouraging residents from accessing Medicaid by way of process burden.
Overall, Medicaid and the Children’s Health Insurance Program (CHIP) saw an increase in enrollment starting in March 2020 and continuing today, though with a slower pace, after at least 2 years of decreasing enrollment, according to a Kaiser Family Foundation report. The same report shows Medicaid program enrollment has increased by about 20% - to about 81 million people – since February 2020 and expects many remain on Medicaid and CHIP rolls as a result of economic uncertainty and instability.
At the intersection of Medicaid, COVID, and economic uncertainty are vulnerable communities, experiencing some of the highest rates of viral hepatitis and HIV. A tertiary benefit of Medicaid’s maintenance of coverage through the public health emergency is those living with viral hepatitis and HIV have been able to more readily seek coverage and care. The problem is a complete lack of “warm hand-off” between Medicaid programs and other assistance programs clients could be significantly advantaged by. Particularly, because of the overlap in intersections of oppression and risk (which some more readily recognize as “social determinants of health”), AIDS Drug Assistance Programs, Ryan White services, and other support services (both publicly and privately funded) are critical tools in our public health safety net.
Tossed off the front burner of public health efforts, “Ending the HIV Epidemic” activities have still been chugging along throughout the COVID-19 crisis. The only other concurrent running pandemic didn’t suddenly go away because COVID-19 came rushing to the forefront of our public health efforts. One of the things these other support programs struggle the most with is ensuring the public (and even health department and hospital case managers) know these programs exist. State Medicaid programs, AIDS service organizations, Ryan White Clinics, and all other safety net programs should be coordinating for the shift in patient load across appropriate programs now. These planning activities should not wait until the midnight hour. For county run COVID-19 testing sites and vaccination sites should be providing information to every, single person seeking a vaccine about available programming to meet the needs of community members. From rental assistance to food pantries to ADAPs, programs already reaching communities and families are the most ideal for starting the process of maintain care after the PHE ends. That starts with passive efforts like brochures and should continue with more active efforts, like engaging a state’s 311 information system with linkage to care tools, and more active still by employing navigators at the Medicaid level to assist clients in finding services, should those clients find themselves ineligible post-PHE.
While we’re not there yet (and it make take significantly longer than any of us like due to a lack of equitable global vaccine access and variant development), advocates, states, service providers, and patients should be planning for what comes next when the PHE eventually comes to an end. We cannot afford to lose people to care at this critical juncture.
Degrees of Separation: Social & Spatial Networks of HIV & HCV
In 1929, Frigyes Karinthy posited a theory many of us might attribute to Kevin Bacon: everyone on the planet is but six degrees of separation (or less) from one another. Depending on how one would measure a connection, that metric is likely far less than it was in 1929. Beyond social media marketing, connecting these networks of friends and friend-of-friends has been pretty important to the concepts of “partner testing and notification” utilized by disease intervention specialists to disrupt chains of transmission in terms of STIs and HIV. What’s been less well understood is the geographic relationship between areas experiencing outbreaks or “clusters” or the specific venues in which transmission occurs – social-spatial networks.
A study performed in New Delhi sought to better understand the relationship between social circle and gathering venue among “hard to reach populations” homeless people and/or, particularly, people who inject drugs. Originating recruitment from within community, asking community to propel recruitment, and paying particular attention to the “mutuals” between otherwise unconnected participants, the researchers sought to better understand the relationship of “risk” of transmission not just in behavior or large geographic area but in specific places in which specific behaviors are part of the culture – the community standards, if you will - of that venue. Researchers found 65% of participants had HCV antibodies, of which 80% had an “active infection” and most were unaware of their HCV status. Similarly, of those participants living with HIV, 65% were directly connected with another participant living with HIV. Researchers did not specify these connections to be causative – those connected did not necessarily transmit either virus to one another. Further, researchers found partaking at the most popular venue was associated with a 50% greater likelihood of a participant having an HIV or HCV diagnosis. Even if a participant did not access the most popular venue, if they associated with someone who did, their likelihood for being diagnosed with HIV or HCV was 14% higher. And the more degrees of separation a participant had from someone who accessed the most popular venue, the less the likelihood of a diagnosis.
The researchers conducting the study were hoping to identify methods of understanding that would allow for effective interventions that reach beyond the individual level. Can group behavior be influenced beyond recruitment and toward changes? Can harm reduction strategies or housing programs find greater efficacy, a better stretch of our dollars, by better understanding where these networks exist and how they operate?
Or is this association merely a by-product of sharing certain characteristics society has deemed unworthy of care? Those social ills that drive disparities in health and poverty and addiction may also drive those experiencing these harms of bias and negligence to seek a social network that at least understand their struggles. To be a little less alone in these struggles.
As is the nature of most things, a better understanding of behavior doesn’t always lend itself to building positive interventions. The same ability to navigate networks in areas where people living with HIV are discriminated against and people who inject drugs can easily be criminalized, rather than connecting to care. With molecular surveillance generating the ire of HIV advocates over fear of this kind of detailed knowledge being used by law enforcement, advocates should also keep a keen eye on how networks may be weaponized as well.
Understanding the spatial relationship within a social network could be a powerful public health tool that shifts our focus from individual intervention to far more meaningful interventions, so long as we can keep the focus of this type of research and the information gathered from it squarely aimed at building up the “public” part of public health rather the continuing to push the responsibility of public health on individual behavior.
Potentially Powerful Tools: A Vaccine in the Fight Against HCV
In 1989, Sir Michael Houghton helped discover the Hepatitis C Virus. Last year, he was awarded the Nobel Prize in Medicine for this same discovery. Now, he’s aiming to create a vaccine against the virus.
Just a few weeks ago, Houghton announced an effort at the University of Alberta’s Li Ka Shing Applied Virology Institute that could have a adjuvanted recombinant vaccine ready for global deployment by 2029. Both new mRNA and viral vector vaccines, used in the COVID-19 vaccines currently authorized by the Food and Drug Administration under an emergency use authorization, have the potential strength to produce multiple kinds of antibodies, solving a long-held problem in the search for an HCV vaccine.
A couple of words of caution: A recent study, using two viral vectors, while successful producing HCV specific T-cells, failed to prevent chronic HCV infection. The last decade has seen several attempts at developing an HCV vaccine but few have made it to human trial, specifically because of evasive properties of the virus’ genotypes to behave differently or escape the body’s natural defenses.
If Houghton and his team are successful, a 2029 launch would likely still have much of the globe well behind the World Health Organization’s 2030 goals but adding a clear, definitive prevention tool stopping chains of transmission would, in theory, help countries playing “catch-up”. With complications from Hepatitis C killing more than 400,000 people annually – and possibly more in the coming years due to COVID-19-related disruptions in care – a vaccination effort could very easily save millions of lives and save billions in public health and health care funding. Houghton suggested Canada alone could see a reduction in HCV-related costs of 98%, or $20 million as opposed to $1 billion – annually due to the high costs of direct acting agents, which are the current gold standard in HCV care and can be curative.
However, if global access to COVID-19 vaccine difficulty and notable vaccine distrust and failure to uptake have taught us anything, having the technology is not the same as having the technology. COVID-19 doesn’t appear to be slowing down, despite recurrent waves, and supply demand on shared vaccine ingredients could find HCV deprioritized.
Additionally, there’s other potential complications to consider. While the United States has seen an increase in Hepatitis B vaccine acceptance, in part thanks to an infant vaccination schedule inclusive of HBV, a study published last year found waning immunity over the years post-vaccination. Now, the CDC’s information page does not recommend HBV boosters and technology may differences may naturally boost efficacy of vaccines but anything that needs additional follow up, like multi-stage vaccines or boosters, are always prone to follow-up failures. And – yet again – COVID-19 provides an excellent case study in this (and all other hidden asterisks). With “anti-vax” sentiments rising at exceptional rates, especially associated with “new” technologies, adding yet another vaccine to the schedule may find barriers in acceptance other common vaccines haven’t run into, at least not on this scale.
Nevertheless, a successful HCV vaccine that answers the challenges of the virus, the conspiracy, and the supply would be a game-changer (to use an unfortunately over-used phrase).
If advocates and policy makers have learned anything, being well prepared for an ever-changing environment in terms of technological advancements, the advancement of anti-science sentiments, and a whole host of other challenges is to our benefit. Analyzing the years between now and, hopefully, 2029, perfecting routine vaccination programs and ensuring actual global equity in access and distribution are critical to making sure this kind of discovery doesn’t go to waste.
Jen’s Half Cents: The Trauma of Advocacy
Author’s note: This blog will not be giving any identifying information, screenshots, or links to provider or advocate comments. As the focus of this blog is a need to acknowledge the traumatic nature of working in public health and advocacy, protecting the identities and personal spaces of these heroes is of the utmost importance.
In April of this year, a friend I’ve worked closely with on a variety of personal and professional levels wrote me: “So…I’ve quit my job and left the field.”
They had spent years working for a health department funded entity serving both urban and rural areas, doing outreach, contact tracing, care and coverage navigation, advocating for clients with providers, for providers with administrative management – the full gamut of public health activity, specifically around HIV and STIs. Last March, they were sidelined to COVID work, face-to-face, often without protective equipment, lacking time off, enough staffing support, and more. Everything everyone else went through as COVID gripped the nation with the most stringent mitigation efforts. Over the summer they would be tasked with pulling “double duty” between COVID contact tracing efforts and HIV and STI work.
This story is not unique.
While higher profile exits from the field of public health have gotten some attention, as evidenced by CNN’s coverage in May on more than 250 public health officials having quit, resigned, been fired, or retired, those that take on the daily tasks of providing care within communities haven’t gained the attention they deserve. In January, the National Coalition of STD Directors published their “Phase III” survey of state STI programs and the findings were startling – every, single published comment discussed the issue of burnout among disease intervention specialists, data managers, and other client-facing staff. As the so-called Delta Variant of SARS-CoV-2 grips the nation in now a noted “fourth wave”, these human resources making the very body of public health haven’t been replenished. Many providers, advocates, community members, survivors, loved ones of those lost to COVID, some elected officials, and those at greatest risk of severe COVID complications or loving those who cannot yet access vaccines have taken to social media to voice their frustration with the state of the nation’s response to COVID-19.
For HIV/AIDS advocates, this isn’t new. We’ve spent four decades being ignored or actively discriminated against, having our stories stolen from us and mutilated in efforts to demonize us, our vulnerabilities and very disease state criminalized – used as justifications for denying us basic freedoms and access to the very care that keeps us and our loved ones alive. We’ve watched promises made, lofty goals announced, and the dollars behind those goals go unused due to lack of flexibility and then usurped to put children in cages and concentration camps, those dollars used to rip children from their parents, sometimes right before their eyes. People living with HIV accessing Ryan White programs are asked to detail to case managers intimate and personal aspects of their lives they may never share with other people. The same case managers who are over worked and underpaid and can’t be provided the supports they need in order to make clients feel like the ears and eyes prying in their lives actually care.
Yet and still, these same voices, these same lives and experiences are those relied upon to move legislators and policy makers, and beg and plead for changes that would reduce barriers to care for us and other people. There is not a single advocate I know, personally, who has not run into a barrier to care or system failure or – frankly – a bigot abusing politics or process who has not turned around and fought with every breath to ensure those harms are ended. “I will do everything I can to make sure no one ever has to go through what I went through.” There is a love in this sentiment that cannot be measured. It fills you up – it fills me up – from your gut to your chest, it becomes the wind at your back and that love inspires and sustains…for a while. That love stands in stark contrast to the politicized and polarized response to COVID-19 mitigation efforts and vaccination campaigns where frustrations run up against conspiracy theories and near sociopathic adherence to contrarian conflict.
We don’t talk about what it is, the personal cost, to retell our stories time and again. We don’t talk about the nature of purposely reengaging our traumas in order to advocate for the world around us. There’s a fear that runs quiet in the background when some decides to change their path or step back from advocacy. That fear often sounds like a hushed phone call, “Am I a bad person for not being up for this?” All that fear compounds with the daily stress of paying bills or commuting or caring for family or going to school so you can be heard with more legitimacy or…or…or….
That piece is the emotional labor of survival.
Advocacy and public health are not for the faint at heart. And….
Those entities, governmental and private, funding care and advocacy, regardless of space – be it oncology, HIV/AIDS, STIs, substance use recovery – need to consider these costs when evaluating awards. When compensation for these stories or “community engagement” often tops at twenty dollars an hour, funders are telling those with the courage and voice to share those stories that our years of trauma – the very expertise of “lived experience” or existing at the intersections making up your consumer base – is worth less than the average cost of your tank of gas. Supporting communities means supporting a living wage, supporting operations costs, supporting expanding staffing, supporting entities with mental health days as part of leave policies. Supporting effective advocacy and efficient public health means supporting the very humanity behind these efforts.
WHO Hepatitis C Elimination Goal Slipping Away
In 2016, shortly following the introduction of direct acting, curative agents, the World Health Organization set a goal of global elimination of HCV by 2030. At the time, it was an ambitious but potentially achievable goal. The WHO, much like the rest of the world, couldn’t have anticipated declaring another pandemic in 2020 and its massive disruptions to screening and care. Now, as the world trudges through limited COVID-19 vaccine access, outbreaks, and variants, public health experts are returning to familiar concerns while still wading through COVID-19 and seeing a worrying trend in the strains caused by the pandemic.
The WHO estimates about 71 million people living with HCV with about 400,000 people dying annually due to liver failure and hepatocellular carcinoma caused by the virus. Despite the WHO’s lofty 2030 goals, those numbers are growing, not decreasing. Unfortunately, that trend isn’t new.
Sarah Blach, of the CDA Foundation in Colorado, recently evaluated and updated modeling from her team’s 2017 projections under the lens of COVID-19 disruptions and the results were startling. Prior to diving into the forecasting data, it’s important to note Blach’s team found HCV screenings and treatment initiation were declining even in high income countries. Prior to COVID-19, only 5 countries out of the 110 included in the 2017 model were on track for their 2030 elimination benchmarks. The model discussion cites both the United States (with a 60% reduction in patients receiving treatment in 2019 compared to 2015) and Italy (with a 35% reduction in treatment initiation from 2018 to 2019). Blach’s modeling team provided estimations considered under a “no recovery” model, “no change scenario” relative to 2019 data (or a 1-year delay in screening and treatment uptake model), and an “escalation” model in terms of efforts to eliminate HCV – particularly among lower-middle income countries. The model found that, if the United States were to resume static screening and therapy initiation efforts at pre-pandemic levels or exceed those goals, between 10,000 and 25,000 cases of hepatocellular carcinoma would be averted from 2021-2030. Though, this model would require treating almost 250,000 people per year in order to reach the 2030 elimination goal – in context, the United States initiated treatment for just 150,000 people in 2019 (also missing the targets for the year by 10,000-20,000).
Blach’s modeling uses the United States as a frequent example because, despite being a high income country, not much in the way of incremental incident infections were expected because the United states does not largely engage in best practices related to (and some would argue “required to”) HCV eelimination (ie. fibrosis requirements related to DAA initiation, care rationing, and restrictions on treatment access for people who inject drugs. The model found under a 1-year universal delay of treatment initiation, no country would achieve HCV elimination by 2030, particularly because the year of delay in treatment initiation doesn’t stop transmission of HCV and considers many lower-middle income countries may experience additional barriers to trust and patient engagement, potentially delaying timely diagnosis for years. Limitations to the model include a universal calculation of delay and local holidays and customs may more readily adapt. The most significant limitation in the model is it does not consider the impacts of increased risk behaviors or decreased access to harm reduction programs. Blach and team use the closing moment to ask policy makers to consider the role of investigating and investing more in HCV screening and treatment efforts in order to not lose critical, fragile ground in the fight against HCV. Given a different study’s conclusions on exactly when particular states in the US might reach their elimination goals with only 3 (Connecticut, South Carolina, and Washington) making it in 2030 and several others’ efforts taking until after 2050. The same study notes, of the states with the most restrictive access to HCV care policies and lack of harm reduction infrastructure, theirs are the ones estimated to take the longest to achieve HCV elimination.
2020 did not hold all bad news for the fight against HCV though. Nature covered 3 of the most impressive study efforts in HCV, with findings presented in 2020 and 2021, which may hold some of the keys to reducing HCV-related deaths and improving other health outcome goals. Egypt’s shift from a national treatment program to a national screening program, amid the treatment program being so successful the need to identify HCV patients more readily, also modeled effective cost savings opportunities as $85 USD per patient to identify an HCV infection and $130 USD per curative course. A Scottish study found particular pharmacies were more apt to identify patients at higher risk of HCV for screening, more likely to successfully initiate treatment, more likely to maintain program of care, and more likely to achieve curative status than more traditional physicians. Lastly, a third paper found – in a limited study - prophylactic therapies could allow countries to expand organ donation access by way of making HCV positive donor material safe to provide for non-sero positive patients. As organ donations are strained, this type of progress could enhance care and outcomes among solid organ transplant recipients.
While there’s promising opportunities on the horizon, we cannot lose sight of the fact 400,000 people a year die HCV-related complications. In a year in which much the of the United States is grappling with understanding the weight of half a million deaths, the global toll of a similar number might get drowned out amid the noise of COVID. We can’t afford to leave behind these noble goals or our global partners in HCV elimination. Like with COVID (and any other infectious disease), it’s not over for any of us until it’s over for all of us. While COVID has gripped the globe and limited access to vaccines makes for a shocking headline, we’re only in the first 2 years of this disease and have made significant, even if unequal, progress. There’s a path. HCV has killed tens of millions over the last 2 decades with equally amazing tools to combat the disease. Yet and still, millions are poised to die due to inaction and lack of sustainable investments to eliminate the virus - in large part because the risks associated with HCV transmission are judged to be “moral ills”. There is no greater ill than standing idly by allowing death to rage through the most vulnerable in any society. COVID may be the mechanism for which we’re having that particular conversation on global equity in this moment and it would behoove us all to remember HCV, a treatable and curable disease, remains a threat.
340B Drug Discount Program: Here’s What Patient Advocates Need to Know
The 340B Drug Discount Program for years has had little attention, aside from a few Congressional Hearings. As we cited last month in a blog, 340B program purchases has more than quadrupled in the last decade, now exceeding Medicaid’s outpatient drug sales. This growth has disturbed the bargain made between manufacturers, providers, and lawmakers in 1992, often leaving patients out of the benefit meant to be gained by the program.
Because 340B is an exceedingly nuanced payment system design, lawmakers have been reluctant to touch the issue – fearing a need to “crack” into the legislation, lacking agreement on how to proceed, and having to balance interests that are often in conflict – preferring to leave the management of issues arising around 340B to the Health Services Resources Administration (HRSA), which then has the unfortunate duty to remind lawmakers, the agency’s statutory authority is limited, and their budget is not large enough for more meaningful oversight. As administrations change, so do the perspectives on how to ensure the intent of 340B, making sure poorer patients can afford and access outpatient medications and the care required to acquire those medications, is captured in how the programs actually operates. Leaving us with the current situation of competing interpretations and interests heading to the court system to find answers and settle disputes.
Part of this program growth is driven by hospitals as a type of “covered entity”; a 2015 analysis showed the program having grown from about 600 participating in 2005 to more than 2,100 hospitals in 2014. In fact, a 2018 Government Accountability Office report found “charity care” and uncompensated care provided by hospitals receiving 340B revenue had steadily been decreasing over the years. The Affordable Care Act has something to do with that – in extending Medicaid eligibility, the Medicaid qualified population grew and as enrollment grew, so did the amount if “disproportionate share” of Medicaid patients certain hospitals served. Ultimately, this meant more hospitals qualified for the 340B Drug Pricing Program than had prior to the ACA.
Another reason for program growth is an expansion of definition of “covered entities” to include contract pharmacies – which have grown as an industry – used by federal grantees like federally qualified health centers (FQHCs) and hemophiliac clinics. Tim Horn, director of the Health Care Access team at the National Alliance of State and Territorial AIDS Directors, described why it was necessary for this expansion, in particular to Ryan White clinics, serving communities affected by and vulnerable to HIV as opposed to limiting program qualification to those pharmacies run and owned by clinics themselves, “340B contract pharmacies are vital to Ryan White and other safety net providers for a couple of important reasons: they help ensure equitable access to affordable medications by uninsured clients, including patients who might live too far from a program's in-house pharmacy, and they help programs maximize their ability to generate essential revenue on prescription fills for insured clients.”
Regardless of entity type, most patients access care through a “payer” (health care insurance provider, be they public – like Medicaid managed care organizations – or private), who play a central role in the 340B payment system design. In turn, this means “pharmacy benefit managers” (PBMs - who sometimes also own the contract pharmacies in question) also play a central role, by designating schemes for how providers are reimbursed for care they’ve provided or medications that have already been dispensed. Jeffrey Lewis, a board member of Community Access National Network and President & CEO of Legacy Health Endowment, described how some PBMs engage in discriminatory practices by paying for 340B drugs at lower rates than non-340B drugs, reducing the benefit Congress intended to give 340B hospitals and clinics:
“340B providers receive less revenue than if 340B drugs are reimbursed at normal non-340B rates. That loss of revenue results in 340B providers having less money to underwrite the cost of providing uncompensated care, including serving uninsured or underinsured patients or providing services that insurers do not reimburse. PBMs, on the other hand, retain the difference between the 340B and non-340B payment rates for themselves. This program "benefit", which was intended to go to non-profit safety net providers, ends up going to for-profit PBMs instead. In this manner, PBMs' payment policies prioritize PBMs’ for-profit interests over 340B providers' non-profit missions to support public health.”
The center of one of the most pressing actions to date is “who’s job is it to make sure the rules are being followed?” with manufacturers being the first to move – by way of seeking the ability to require entities wishing to participate in 340B to provide additional claims data. Lewis points out that in a unanimous Supreme Court decision in 2011, courts had previously interpreted covered entities as lacking authority to seek enforcement against manufacturers, so the same must be true in reverse, requiring all parties to use a dispute resolution process dictated by HRSA. Indeed, the ruling even goes so far to cite the ACA’s directive for HRSA to issue a formal “alternative dispute resolution” process. However, HRSA failed to formalize this process in a final rule until December 2020. That rule is now part of a patch work of suits from manufacturers looking to the courts for clarity, with manufacturers arguing that statutory enforcement can’t be one-sided – if manufacturers must provide these discounts, someone should be ensuring the entities receiving these discounts are actually using them for patients and HRSA, by their own admission, doesn’t have the capacity to do so. Of note, Justice Ginsburg, who pinned the 2011 ruling in Astra USA, Inc., noted HRSA’s failure to bilaterally enforce the rules did not necessarily provide for a right of action by 340B actors.
Nonetheless, 340B remains a critical source of revenue for Ryan White clinics and other federal grantees already meeting the legislative intent of the program, at least generally better than other payer and provider actors in this scheme. As a result of sustainable federal funding and legislators prioritizing public health funding, federal grantees are scrambling – and manufacturers should consider how best to not harm the “good guys” in what ever actions taken next. Indeed, NASTAD’s Tim Horn stated:
“340B program revenue will always be an important – and dynamic – supplemental funding source for our HIV care programs, particularly where Medicaid has not been expanded and where federal and state funding is both limited and inflexible. A number of factors that have real or potential impacts on 340B…are now requiring serious discussions regarding the sustainability of program revenue generation. Simply put, we're not going to end HIV as an epidemic without significant and nimble funding required to support the myriad medical and support services associated with the best possible health outcomes. 340B revenue is a substantial part of this and, absent alternative funding streams to ensure that these programs remain whole, will remain the lifeblood of HIV service delivery in the United States.”
Legacy Health Endowment’s Jeffrey Lewis agreed:
“The value and importance of the 340B program are well known. However, where there is ambiguity, it impacts both covered entities and patients. With the positive growth of covered entities to serve more people in need, Congress must take a thorough look at why 340B was created, its absolute value and tackle the tough questions where ambiguity may exist. Clarity is needed now more than ever to stop pharmaceutical companies from indiscriminately deciding whether and how to participate and prevent jeopardizing patients' lives. Similarly, Congress has an obligation to evaluate the role of PBMs and Third-Party Administrators (TPAs) operating in the 340B space and set a specific rule regarding revenue sharing. The 340B program was created to aid covered entities in serving more people in need. Unfortunately, every dollar taken by PBMs or TPAs reduces the ability of covered entities to care for more and more patients.
Clear legislative intent and rules are critical to ensuring program stability and, ultimately, safety net provider stability. Ryan White Centers, Hemophilia Centers, FQHCs, and rural hospitals as particularly vulnerable to Congressional, HRSA, and OPA ambiguity. The current and future failure to clarify the uncertainty of the 340B program jeopardizes patients and the financial stability of covered entities.”
While the finger-pointing on “who’s at fault” for an unsustainable program growth rages on and works its way through both the courts and the minds of lawmakers or who is responsible for drawing the lines in which manufacturers, providers, and payers can color inside of, the only thing clear is the population this program is meant to serve is not receiving as much benefit from the program as it should. We could say “patients” here, but that word apparently needs to be defined with regard to 340B. In the end, all stakeholders, outside of lawyered language, know exactly who has been harmed by bad actors in the 340B landscape. Everyone with power in this minefield would do well to remember that.
We invite you to download the 340B Final Report, issued by the Community Access National Network’s 340B Commission.
Community Roundtable Emphasizes Impacts of Covid-19
In late June, Community Access National Network hosted a virtual Community Roundtable on Covid-19’s Impacts on HIV, Viral Hepatitis, Sexually Transmitted Infections, and Substance Use Disorder. CANN’s policy consultant (yours truly) was joined by A. Toni Young, founder and executive director at Community Education Group, and Kenneth Westberry, senior manager of policy and government relations at the National Coalition of STD Directors, in discussing the wide-reaching impacts of the Covid-19 pandemic and subsequent public health emergency on the nation’s longest and most well-funded public health service providers…so far. Attendees included representatives from patient advocacy organizations, state and local health departments, clinical laboratories, hospitals, pharmseutical companies, and federally or state funded service providers from 20 states and the District of Columbia. The event was sponsored by ADAP Advocacy Association, ViiV Healthcare, Abbvie, Merck, and Janssen Pharmaceutical Companies of Johnson & Johnson.
Toni started off a whirl wind of information with making direct comparisons between the previous year’s overdose death rates and this year’s and emphasizing the plight of West Virginia by comparing the nation’s increases to the state’s. This opened the roundtable with a clear message that would ring through with every new data point: the pandemic’s impacts are not equal. Building upon the point made in a blog post earlier this year, Toni pointed to a stark decrease in HCV screening and, more pointedly, reviewed available data on HCV medication access – showing a decrease of 37-48% during the first few months of the public health emergency. She warned listeners not view initial lower incidence rates as optimistic, rather these findings should be viewed under a lens of a lack of access to screening and services. She further stressed the lack of SUD services accessed at the beginning of the pandemic resulting in alarming increases in injection drug use-related HIV diagnoses as a year over year trend with 2021 looking even more worrisome. Rounding out this segment of the roundtable, Toni cautioned attendees: we have good reason to believe screenings will not necessarily return to their pre-pandemic levels in a speedy fashion or without additional effort and funding.
I followed Toni’s dynamic presentation, picking up with the Centers for Disease Control and Prevention surveillance reports for 2015-2019 – reminding the audience federal level data often lags by two years and the CDC has already presented data for 2020 on fewer HIV tests being performed. This portion of the presentation highlighted disparities in HIV along geography, racial and ethnic lines, as well as sex assigned at birth. I needed to note: gender identity is not uniformly collected data in HIV surveillance. The CDC’s pre-exposure prophylaxis data was similarly…unfortunate. With right around 10% for Hispanic/Latino people identified as living at risk for HIV receiving PrEP services and medication in 2018 and just over 6% of African American/Black people living at risk for HIV receiving PrEP services and medication in the same year. Similarly, people assigned male at birth were more likely than people assigned female at birth to have access to PrEP. Looking to the pandemic, I cited two Kaiser Family Foundation reports one on the similar disparate impacts between HIV and Covid-19 among racial and ethnic communities compared to their white peers and the other on Covid-19’s impact on Ryan White service providers. The KFF reports showed service providers reporting an increase in patients without insurance or receiving Medicaid, some clinics reporting a decrease in patient retention and other reporting increases in patient retention, and clinics reporting a decrease in patient demand for HIV screenings and accessing PrEP services.
The final presenter, Kenneth Westberry, began by giving a brief overview of the state of STI’s as public programming: a steady increase year over year in reported STI incidence, a lack of significant funding increases in the last 15 years, and nearly 40% of clinics reporting a decrease in hours or closing entirely during the height of Covid-related restrictions. Of the particular burdens, Covid-19 brought state and local health departments, nearly 80% redeployed their staff from STI programming to Covid-19 programming, reducing capacity to manage STI caseloads, and facing an unprecedented lack of testing supplies as manufacturers also refocused on making Covid-19 tests. Kenneth then reviewed the findings of NCSD’s surveys seeking to evaluate the state of STI programs (phase I, phase II, and phase III) showing many health departments are still behind in terms of having enough staff to meet the needs of both Covid-19 as a public health emergency and regular STI programs.
Moving onto the nuts and bolts of the federal response to Covid-19, Kenneth highlighted the role of disease intervention specialists historically and in response to Covid-19, answering the “why” the Biden Administration’s change in stature toward the pandemic was critically necessary. Particularly, the American rescue Plan Act added $1.13 billion to expand and sustain current DIS and the President’s budget request includes an increase in funding for STI programs in addition to current spending levels.
The three panelists then spent a brief amount of time discussing the funding weaknesses exposed by Covid-19 diverting resources. In a particular “shot across the bow”, Toni stated “Health departments and appropriators have learned Ryan White dollars aren’t sacrosanct anymore. If the emergency is big enough, they can grab those monies,” urging advocates to keep on their toes and watch actions at the state and local as much as they do at the federal level. Each panelist also mentioned a need for greater collaboration between “silos” in order to reach the nation’s lofty public health goals with regard to HIV, HCV, STI’s, and SUD.
Panelists wrapped up by highlighting upcoming events for each organization, sharing resources, and once again thanking each other, attendees, and sponsors. The slide deck can be downloaded here.
Future events will be hosted to ensure we’re “tracking what’s on the ground” and connecting community partners with pertinent resources and information.
Emergency Alert: Substance Use Safety Net in Trouble
In April, the Biden Administration released a Statement of Drug Policy Priorities, outlining areas of improvement and policy priorities necessary to address the nation’s opioid epidemic. The statement was followed up in May by the Substance Abuse and Mental Health Services Administration (SAMHSA) announcing $3 billion in block grants to be distributed to states as emergency funding related to the American Rescue Plan Act, passed in March. The press release from SAMHSA highlighted preliminary data from the Centers of Disease Control and prevention (CDC) showing an estimated 90,000 overdose deaths in 2020, a 20,000 overdose death increase from 2019. Belying the nature of “emergency”, the SAMHSA announcement came 2 months after President Biden signed the American Rescue Plan Act, the release did not include a timeline for these block grants to be received by states or any indication from SAMHSA that guidance attached to use of these funds would be forth coming. That’s a problem for substance use treatment service providers who have been struggling to keep their doors open and services flowing throughout the COVID-19 public health emergency.
According to Michael Pickering, executive director for Regional Addiction Prevention, Inc. (a residential treatment program in Washington, D.C.), residential programs had to cut the available number of patients who could be housed at any given time, in order to help slow the spread of COVID-19 and reduce risks of clients and staff contracting the virus while restrictions were in place. That meant revenue necessary to operate shrunk dramatically. However, in order to maintain a minimum number of services, not many staff were reduced. Ultimately, these combined factors underscored a long-term issue as the agency faces potential closure: private and public payer rates for are so low that even the slightest emergency could be catastrophic for many substance use service providers.
Indeed, the Centers for Medicare and Medicaid Services (CMS) last adjusted addiction treatment services reimbursement rates for inflation in 2016 – meaning the “basement” for reimbursement hasn’t increased in 6 years while the rest of costs associated with providing care have grown. According to one entity, CODAC Behavioral Healthcare, reported reimbursement losses of nearly 40% from Medicaid on a 45-minute treatment session. The same report cited the American Society of Addiction Medicine (ASAM) as stating Medicaid as the largest payer of medication assisted treatment (MAT) for opioid use disorder, accounting for anywhere between 35 and 50% of services provided in the hardest hit states.
Tucked among Biden administration priorities in drug policy is a familiar statement regarding racial equity in health care. At the intersection of disparities in access to care and lack of health equity along racial lines is Medicaid’s low reimbursement rate. A study, included in the Statement, specifically highlighted how few private providers accept Medicaid for substance use treatment, opting instead for “cash-pay only” policies, resulting in a concentration of services provided to white people and leaving an unmet need among people of color, who have also been disproportionately impacted by the COVID-19 pandemic.
While the Biden administration also cited a need to recruit and retain medical providers and staffing talent to stigma, low reimbursement rates also translate to low compensation relative to other areas of health care and substance use services require more time to functionally provide for the needs of a client than other areas of health care. This leaves clinics and providers serving the public with an exceedingly high turn-over rate; CODAC cited a near 50% turnover rate for 2019. Just as with federally qualified health centers, compensation rates tied to clinic revenue (reimbursement rates) and grant awards that aren’t meaningfully increased, can’t compete in terms of compensation – the private sector, focused on profit margins and serving well-to-do clients, can readily recruit skilled talent from public service entities with more attractive compensation packages.
The administration’s priorities in drug policy are lofty and admirable, with a comprehensive map on moving forward. However, getting these resources – especially emergency funding - to entities providing critically necessary services seems to be a major barrier the administration doesn’t seem to have a good plan to deal with. Just as with the emergency rental assistance monies allocated under the American Rescue Plan, emergency monies are slow to reach substance use service providers in dire need, risking destabilizing an already weak safety net. Regional Addiction Prevention, Inc. provides just one example of an entire industry at risk, where funds are bottlenecked at the local agency distribution level. Earlier this month, advocacy organizations from around the country and service providers in D.C. wrote to the Mayor and City Council urging an expedited process to ensure emergency funds for these services were distributed in a more timely fashion.
The Biden administration can set all the goals in the world and even secure funds from Congress, but these goals won’t be met on a one-time funding or periodic “emergency” funding basis. The administration needs to provide funding distribution guidance commiserate with the urgency of keeping public addiction service providers afloat, ensure the country’s annual budget reflects these priorities, and increase Medicaid reimbursement rates to reflect these policy priorities.
Deceptive Masks: COVID’s Threat to STI Surveillance
In April, the Centers for Disease Control and Prevention (CDC) released its annual sexually transmitted infections (STIs) surveillance report, reflecting an increase in overall rates for the sixth year in a row, with a nearly 30% increase in STIs from 2015 to 2019. While sharpest increases in incidences were of syphilis among newborns, the infection burden is not equal with young (ages 15-24) people, gay and bisexual men, and people of color facing exceedingly disproportionate diagnoses. What’s important to note is traditional CDC surveillance reports lag by about two years – these data do not account for COVID-19 impacts among screening and treatment of STIs.
In the report’s press release, the CDC acknowledged COVID-19 posed extreme threats to screening, treatment, and prevention, as public health programs and staff typically used to address STIs had largely been repurposed in response to COVID-19, citing a survey from January showing about one third of local and state health department STI staff were still deployed to COVID-19 activities. Shortages also include screening supplies, according to a September 2020 “Dear Colleague Letter” with regular updates posted on the agency’s drug and diagnostic test notices page showing marginal improvement as reported by testing kit and supplies manufacturers.
The aforementioned survey of local and state health departments was conducted by the National Coalition of STD Directors (NCSDDC), “a national public health membership organization representing health department STD directors, their support staff, and community-based partners”. While NCSDDC usually throws most of its resources into advocating for public health policy changes, funding, and offering technical assistance, throughout the COVID-19 public health emergency, NCSDDC has found itself in the unique position of reporting on the situational needs of health departments and their staff, tasked with meeting a multitude of needs in any given community. The organization summarized its Phase III survey results as follows:
“This continued diversion of staff and other resources has caused delays in providing disease intervention services, leaving some STDs completely unchecked. STD programs continue to report clinic closures, reduced clinic hours and services, STD testing kit shortages, and diminished laboratory capacity. Additionally, STD programs report severe burnout as disease intervention specialists (DIS) pivot from COVID-19 investigations and contact tracing back to STD disease intervention and partner services work.”
For context, NCSDDC, in March of 2020, initially phrased the state of local and state health departments responding to COVID-19 as a “starved public health system in distress”. An indication that despite pledges from the White House and billions in funding allocated by law makers, “on the ground” not much has yet changed for the first responders of public health.
Complicating matters, some health officials are debating the implications of initial surveillance reports for 2020 seemingly showing certain decreases in STI diagnoses, according to one news report, as either a reduction in sexual activity among at risk persons during stay at home orders or a lack of screening. Given the context of reduced capacity, staffing, and supplies, entertaining the possibility of decreased sexual activity rather than decreased access to services shifts the responsibility (and pressure) on state lawmakers and executive offices to appropriately fund and support public health programs to that of undersupported health departments, contracted service providers, their staff, and the vulnerable communities they serve.
As discussed in HEAL blog posts from earlier this year, COVID-19’s impact on public health activities is still being discovered, largely through emerging surveillance gaps (lack of screening) and, as the CDC’s STI report shows, at a lag of data rather than a decrease of incidence, leaving communities vulnerable to outbreaks.
Later this month, on June 30th, NCSDDC will be joining Community Access National Network and Community Education Group for a virtual Community Roundtable on COVID-19’s impacts on HIV, HCV, STIs, and substance use disorder, providing stakeholders and advocates a space to further explore where public health efforts have been strained and can be strengthened in light of COVID-19.
Mr. Becerra, Bring Back the Mega-Guidance. With Love, 340B
Last month, President Biden released details of his proposed budget, including a much needed 23% increase in discretionary spending for the Department of Health and Human Services. Among numerous proposals for these funds, including refilling supplies in the National Strategic Stockpile, expanding mental and behavioral health services, and advancing Ending the HIV Epidemic initiative, is a giant but quiet drug rebate program: 340B. Outside of health policy “wonk” circles, 340B doesn’t often get very much attention. However, inside of those circles an obsessive chant can be heard, “bring back the mega-guidance”.
Let us back up some.
In the early 90’s, another time when drug prices were nearing the height of health care conversations on the national level, Congress and pharmseutical manufacturers struck a balance: in order to ensure a manufacturer’s products were available to a ready purchaser (Medicaid, Ryan White, and other safety net programs), manufacturers would offer their products at exceptional discount based on use – or a rebate. The dollars received as rebates were expected to be used “to the benefit of patients”. The idea being a system-oriented effort to ensure poorer patient populations could get both the medicines and the care they need. Later, the definition of eligible entities for these discounts were expanded to include entities who were not necessarily federal grantees or subrecipients.
The problem, nearly 28 years later is regardless of Congressional intent, no one has definitions for what any of this means. Manufacturers argue non-federal grantee entities (including for-profit hospitals, contract pharmacies, and pharmacy benefit managers) are abusing the program and not just to the detriment of their own interests but to the detriment of patients by way of narrowed provider networks, skewing formularies, and buying up competing practices as examples. Indeed, these are the very examples Senators cited in 2018, the last time 340B was meaningfully discussed on the Hill. Senators then listened as representatives from the Government Accountability Office (GAO) and Health Resources Services Administration (HRSA) see-sawed between saying they either lacked the statutory authority or merely lacked the proper funding support to shore up to program, eliminate abuse, and more adequately perform audits.
The issue at hand was the result of the then-new Trump administration torpedoing the Obama administration’s “mega-guidance”, which would have defined “patient”, provided for a federal portal of products captured under 340B to extend transparency, and more. For context the Obama administration had given notice in 2010 of proposed rulemaking. Which ultimately didn’t manifest an actual proposed rule until August 2016, by which time it was too late to finalize as is the tradition for incoming administrations to pause or cancel late-made rules of the previous administration.
In that 2018 Senate committee hearing, Senators argued HRSA already had the authority necessary – pointing toward the abandoned mega-guidance – and ultimately came to no conclusions other than “things need to change”. That’s an understandable sentiment given the growth of the program. Even if the general public is to question the data of the Pharmaceutical Research Manufacturers of America (PhRMA) stating provider and pharmacy profit margins from 340B grew more than 900% from 2013 to 2018, instead of sharing those savings with patients. However, even HRSA’s own data now puts 340B at nearly the size of Medicaid’s outpatient drug program, up by 23% from 2018-2019 alone. If consumers considered the idea their medications might have been 23% lower in cost if those savings were shared with them instead of pocketed by other entities in the health care pipeline in one year alone, the rising anger shifts the blame quite readily.
There’s plenty to go around, though. The 340B can has gotten kicked down the road for far too long. In the absence of rulemaking, various players in industry have tried to fill the gaps. Last year, several manufacturers began implementing their own practices, primarily by imposing new, internal requirements on contract pharmacies to prove a patient actually qualified or merely refused to allow contract pharmacies to play middle men at all. HRSA responded by sending letters to 6 of the largest manufacturers implementing these programs and demanding they resume offering the discount program to the contract pharmacies – including a threat to penalize manufacturers who refused to cease these limiting activities. A particular manufacturer, Eli Lilly, sued to stop HRSA from enforcing this threat.
Ultimately, though, HRSA hasn’t been able to meaningfully explore its regulatory powers with regard to 340B. President Biden’s effort to fund oversight of 340B is necessary as market-based solutions are at best messy and slow and apparently needing judicial intervention. With sufficient funding for oversight and enforcement under the President’s proposed budget, all that’s left is the same, repeated call of patient advocates and “wonks” alike from the last 4 years: bring back the mega-guidance.
For a more detailed review of the variety of issues 340B faces, please review Community Access National Network’s 2019 report here.
Rising Morbidity: Viral Hepatitis Co-Infection with HIV and Age All Associated with Increased Rates of Liver Cancer
In February, researchers associated with numerous universities across Canada and the United States published one of the most comprehensive data reviews thus far conducted on the incidence rates of the most common type of liver cancer among people living with HIV/AIDS (PLWHA) and PLWHA co-infected with viral hepatitis. The study reviewed data collected as part of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD), conducted between 1996 and 2015, with clinical data from 109,283 participants. Conclusions from the study were fairly straight-forward: the combination of HIV status (mono-infection), co-infection with viral hepatitis (HBV and/or HCV) and age all correlated with an increased chance of developing liver cancer (hepatocellular carcinoma [HCC]). The hope of researchers, as evidenced in the study’s introduction was to “inform expectations for other regions with a substantial burden of HIV and HBV-HCV coinfection but with delayed cART [combination antiretroviral therapy] scale-up and limited access to viral hepatitis treatment”.
While most research papers wait to include study limitations at the end, I prefer to open with them as prefacing allows for contextualizing data. The first and primary limitation on the review is clinical information reviewed was necessarily from those people linked to care and correlations provided by the data in the study cannot be applied to the diagnosed-but-not-in-care or undiagnosed population. Second, researchers note, information on relevant, individual health factors were missing from significant portions of participants data (example: smoking and drinking habits, natural clearance of HCV, fibrosis score, and HIV exposure risk). Additionally, data collection was not uniform across all participating entities at the time of linkage to care, though a quality analysis was used to help even things out and ensure the integrity of data comparisons. This lack of uniform protocol also included certain sites not administering or participants not receiving HCV or HBV screening. The last, though likely most significant limitation of the study is the data were collected prior to the advent of curative direct acting agents (DAAs) for HCV, and conclusions cannot be made on the potential positive impacts of readily available DAAs.
A limitation not mentioned and data unassessed is any reference between older ART regimens and newer ones, in which toxicity and tolerability is commonly known to be considerably improved with newer regimens. Liver health monitoring is fairly standard, among other relevant patient labs, for PLWHA because of a relationship between ART and liver health. While it’s understandable researchers who generally enjoy significant funding from manufacturers may wish to avoid broaching this topic, not mentioning the issue, even to say “we can’t make any conclusions on cART tolerability and toxicity as an indicator for adherence or risk of developing HCC” misses an incredibly important elephant in the room for researchers, providers, and patients alike.
Instead, researchers chose to focus on cART “eras” (1996-2000 [A], 2001-2005[B], and 2006-2015[C]), in which there’s a positive correlation between age and era; or those aging with HIV were more likely to be diagnosed with HCC. Highest rates of HCC diagnosis by cART area are as follows: A – between 50 and 60 years-old (HBV co-infection with HIV), B – lower end 70-80 (HCV co-infection with HIV), and C – upper end 70-80 (HCV and HBV co-infection with HIV). This data is particularly valuable on its own, however, as the associated risk cohort shift appears to be very closely related to age (ie. those in the upper end of the C “era” are also those to first receive effective cART and the 20-year age gap between the C and A cART eras).
Ultimately, PLWHA were more than 3 times as likely as the general population to develop HCC and more than 20 times more likely to develop HCC if co-infected with viral hepatitis. HCC incidence among study participants fell along rather predictable lines in terms of HIV related clinical monitoring metrics; those with higher viral loads and lower CD4 counts were more likely to develop HCC.
The study’s finding highlight the need for ensuring access to DAAs and HBV vaccines, ready ART uptake upon linkage to care, and strengthening the integrity of AIDS Drug Assistance Programs, Medicaid Programs, and care provided to incarcerated persons – specifically, ensuring the inclusion of coverage of DAAs in these.
Advocates, providers, and patients can review DAA coverage inclusion in ADAPs and Medicaid and harm reduction policies impacting HIV and HCV with Community Access National Network’s quarterly HIV-HCV Co-Infection Watch report.
CMS Sides with the Devil: Insurers’ Co-Pay Accumulators Remain…for Now
The Affordable Care Act (ACA) was revolutionary in how prescriptive statutory language was in ensuring health insurers (payers) covered costs associated with pre-existing conditions, if they accepted even a penny of federal funding. The trade off was a simple theory: “cover more people and their entire health and we’ll make sure you’re still profitable”. There were hundreds of pages of caveats, definitions, incentives for public programs, pharmaceutical research, and regulatory authority passed to state and federal agencies. Everyone got a piece of the pie to the end benefit of Americans for whom health care had been out of reach for the majority of their lives. We would be healthier together by simply providing people the care we need and reducing overall costs. However, as these things go, payers are creative and pay their lawyers handsomely to find ways around that basic agreement. As payers fight to “contain costs”, co-pay accumulator programs are one of the most disingenuous methods to limit consumer access to quality care and pad payers profit margins.
From issues of discriminatory plan design, or making consumers pay the highest cost-sharing for medications which are only used to treat certain conditions like HIV, to limiting provider networks in such a way that a patient requiring a surgery or emergency care results in surprise bills to toxic practices known as “utilization management” (including, but not limited to, abusive prior authorizations and step therapy, also known as “fail first”), payers have paid their lawyers quite well to find loopholes or design new problems in order to maintain their profits. The ACA’s medical loss ratio (MLR) rule, also known as 80-20/85-15 rule (in general requiring 80% or 85% of a plans premiums to actually be used on costs of care or pay back to balance to consumers) has resulted in a startling 2 billion dollars to be paid back to consumers in 2019 alone. But the rule doesn’t necessarily count other income payers can produce by way of cost-sharing or deductible payments, co-pays (a fixed price typically paid after deductibles are met for care and medications), and – now, more commonly – “co-insurance” (a percentage price typically paid after deductibles are met for care and medications) as part of that rule. The result is consumers and those who would like to see us get the quality, individualized care we need are being put on the hook for payers’ greed.
Patient advocacy often has interesting bedfellows. And at the intersection of our care interests and that of industry, pharmaceutical manufacturers have found what can arguably described as a somewhat socialist model by way of patient assistance programs, often enacted as co-pay card or discount programs aimed at directly benefiting patients by taking care of the patients’ share of a medication’s cost. These programs are quite frequently limited by income or if a person is insured. The idea being to make sure the most costly medications make their way into the hands of the people who need them most and can least afford them. In this, our interests as patients absolutely converge with that of manufacturers. We want quality therapies made available to us. However, when a medication “goes generic”, often these programs are no longer available as a less costly, generic medication is preferred by the payer unless a patient fails that particular medication (see: step therapy, “fail-first”). The problem is generic medications are not held to extraordinarily strict requirements for Food and Drug Administration (FDA) approval that brand name medications are held to. Indeed, earlier this year, Vice offered a fantastic explanation of the problem with preferencing generic medications by payers (both public and private) is harmful to patients and why our generics “approval” process is a threat to the health and safety of patients. It’s no wonder, with the lax oversight of generic medications and the offer of payment assistance from manufacturers that patients would want access brand name and newer medications on the market.
One of the most amazing benefits of patient assistance programs is, in theory, because they’re meant to cover the patient’s cost-sharing obligations, these out-of-pocket (OOP) costs should apply to the patient’s deductible and OOP maximums and reduce the cost burden to patients for future care throughout the plan year. Right?
Wrong.
Payers have near uniformly adopted a practice known as “accumulator adjustment programs”, or co-pay accumulators, in which a payer basically says to a patient and a manufacturer “all for me, none for thee”, taking the entirety of the benefit offered by a patient assistance program and not crediting the patient with those funds received against the patient’s deductible, co-pay or co-insurance, or out-of-pocket maximums. To boot, manufacturers have zero control over this practice and often don’t know when it’s happening until a patient complains about the experience. Payers justify this move as “cost-containment” and disincentivizing patients from seeking more costly medications – which translates to newer, more effective, safer medications (go back to the problem with generic approvals above).
So far, the Centers for Medicare and Medicaid Services (CMS), the primary authority in which payment rules are issued from the federal government to payers, have generally made extraordinary effort to ensure protect the interests of patients and those who align with our interest. In the instance of CMS’s newest rebate rule, CMS chose to side with payers for some inexplicable reason. The rule states pharmaceutical manufacturers, not payers, would have to count these direct-to-consumer assistance programs among “best price” calculations, which govern Medicaid rebate price setting or what the government pays for a medication, if a patient didn’t receive 100% of the benefit of the assistance program. Previous rules on what to consider in calculating “best price” were generally limited to prices negotiated within industry movers inside the supply chain, not that of end users. The theory goes like this: “if ultimately this assistance program is paying an insurer’s bottom line and not helping patients, then it should be considered a price you (manufacturers’) negotiated. You were planning for that in setting your prices anyways, right?” Pop quiz answer: wonky negotiations with payers is not what manufacturers were planning on in designing income limited, only-accessible-by-consumers-asking for-it assistance programs. The solution CMS offered was for manufacturers to ensure patients received the intended benefit by requiring patients to pay for a medication up front and then ask for reimbursement – a process that only makes medication access and affordability infinitely more complicated and burdensome for patients.
In the end, CMS decided that in response to an excessively abusive payer practice that disadvantages patients, the answer was to create further barriers to accessing care for patients rather than to reduce them.
Let’s make this real and “back of the envelope” this practice in terms of realized patient experiences:
Monthly Income: $2,583 (based on average US income in 2019 provided by the Census Bureau)
Monthly premium: $304 (lowest cost local silver deductible is $3,400, OOP maximum is $8550, co-insurance is 20-40%)
Absent a public payer intervention, co-pay accumulators might allow a patient assistance program to cover the estimated $600 per month co-insurance would demand for a certain medication, however, I’m not likely to meet my deductible or maximum OOP for the year at all. With local rent costing about $1000 per month, a car payment and car insurance in order to work (there’s no meaningful public transit in the vast majority of the country), food costs, utilities, etc. Even with federal subsidies provided via the health care market place, every month, I’m in the negative. Which means I can’t afford to see my doctor or get my quarterly labs, which means I can’t get my medication in the first place.
However, without the application of a co-pay accumulator, accessing just 3 month’s worth of a patient assistance program would meet my deductible and maximum OOP costs for the year. I don’t have to worry about at least $200 per month in medical costs. And one less financial strain is off my shoulders.
For the vast majority of us, our medications are not a luxury item. They’re not something we can afford to pay for up front and mail-in a rebate request and wait months for. In doing so, CMS not only suggests an increase to the paperwork burden on patients and manufacturers alike, CMS also seeks to increase barriers to accessing life saving medications to begin with.
All to the benefit (read: profit) of payers. So it’s no wonder the trade organizations, Pharmaceutical Research & Manufacturers of America (PhRMA) chose to initiate a lawsuit to halt the implementation of CMS’s backwards and punitive rule.
While patient advocates may spar readily about the role of industry among advocates, we should also recognize actions that align with our own interests on their face. Yes, PhRMA may be leading up this suit - and CMS should listen to the needs of patients, reverse course, and voluntarily pull this rule.
Medicaid Access: HCV Medication Stalls
In November 2015, the Centers for Medicare and Medicaid Services (CMS) issued a stark warning to state managers of Medicaid programs regarding restrictive limits on accessing newly developed and emerging direct acting agents (DAAs) for the therapeutic and curative treatment of Hepatitis C. Since then, Harvard’s Center for Health Law and Policy Innovation (CHLPI) has steadily tracked the three most impactful methods of restricting access to DAAs in Medicaid programs: fibrosis restrictions, sobriety requirements, and prescribing provider requirements.
Briefly, fibrosis restrictions require a patient to have advanced in the amount of liver damage to a specific degree in order to qualify for care, sobriety requirements restrict access to DAAs based on a person’s self-attested stated or clinically documented sobriety, and prescribing provider requirements restrict recognition of “medical necessity” to that of a specialist or with consultation of specialist in order to receive coverage of a particular DAA. CHLPI’s most recent survey of Medicaid programs outlines progress of the policies of restriction by state. As of the date of the survey, 4 states maintain fibrosis restrictions, 13 states require some period of abstinence/sobriety with an additional 15 states requiring a patient to participate in some level of alcohol and/or drug screening and counseling, and 18 sates have some level of specialist prescriber requirements. Additionally, Community Access National Network’s quarterly HIV-HCV Coinfection Watch Report details which states cover which Hepatitis C therapies and DAAs under their Medicaid preferred drug lists (PDLs).
Of particular note, the 2015 CMS notice specifically highlight the practices of fibrosis stage and sobriety requirements as running counter to various provisions under Section 1927 of the Social Security Act. A 2020 legal review by CHLPI’s Phil Waters describes various case law and potential enforcement mechanisms in which to combat these restrictions, which may prove prescient for federal enforcement agencies and advocates alike. Of particular note, Waters argues the Americans with Disability Act (ADA) presents a “novel” approach in addressing the most caustic and immediate barrier to accessing DAAs by Medicaid recipients: sobriety restrictions/abstinence requirements. Waters notes opposition to this method of seeking enforcement may argue such policies “benefit” the class of persons affected by same. While 2018 guidance from the Department for Health and Human Services (HHS) recognizes substance use disorder as a disability, the same guidance specifically exempts people currently using illicit and illegal drugs from the protections afforded by the ADA.
As we referenced in a blog earlier this year, the Centers for Disease Control and Prevention (CDC) Hepatitis C surveillance data indicates an extraordinary increase in new HCV diagnoses relative to the opioid epidemic. Arguably, requiring an otherwise qualified Medicaid client to undergo additional, non-emergency treatment or engage in non-medical activities in order to gain coverage of a live saving therapy is necessarily discriminatory. After all, a particular Medicaid pharmacy and therapeutics committee cannot evaluate the degree of limitations a person’s experience with substance use disorder causes and imposing additional requirements that specifically target this particular is counter to best practices. Indeed, requiring a person to “get clean” before receiving life-saving medical care is the exact opposite of managing substance use recovery. Relieving pressures of medical need, housing, and other negative pressures related to determinants of health are what set people up for success in combating substance use.
In order for the Biden administration to fulfill its promises with regard to combating the opioid epidemic, for states to fulfill their responsibilities under the National Viral Hepatitis Strategy, and to meaningfully address the intersection of these syndemics, federal agencies tasked with enforcement of these rules and Medicaid directors should consult and act in alignment with advocates with lived experience and best practices in combating both the opioid epidemic and resulting infectious disease outbreaks and diagnosis, including HIV and Hepatitis C. Just as with strategic, culturally competent approaches to combatting HIV and STIs focus on sex-positive education and access to resources, including prevention and treatment interventions, barrier reduction is critically necessary in order to succeed in this fight. As it stands, Medicaid programs present one of the best opportunities to ensure and enact meaningful access to care in an effort to eliminate Hepatitis C. These access limiting policies also present the biggest barriers to achieving that goal.
Jen’s Half Cents: Fixing the Broken Patient Advocacy Pipeline
One of my first conversations with Bill Arnold was particularly memorable. I had just started hormone replacement therapy, my beard wasn’t nearly as strong as it is now, and I’m certain I looked like a 16-year-old. In a moment of career transition, I had also recently joined the board of directors for ADAP Advocacy Association (aaa+). The Washington, DC air was warm, indicating a cool evening ahead, and we were taking a break outside during the ADAP Annual Conference. Following a detailed but brief chat about the state of political play around the Affordable Care Act, we went back inside for the next session and ran into Brandon M. Macsata, aaa+ CEO. What ensued was a discussion of strategy to recruit and engage younger advocates in patient advocacy and in particular the space of HIV. I think, in no small part, because I was the youngest board member at the time and because I was one of the newest board members, this “getting to know you” opportunity was also an excellent opportunity to discuss various priorities in advocacy and the current state of the advocacy ecosystem.
It should come as no surprise we independently concluded the health of the patient advocacy ecosystem was weak, having surpassed the moment of crisis, interests in our needs were waning, especially with certain competing priorities, advancements in therapeutics to treat HIV, and chronic nature of the illness that brought us all together. To be honest, the time between then and now, much like the nature of any chronic illness, has not much changed and it certainly hasn’t advanced. Even with the heavy reliance on a variety of expertise from the field of HIV to address, mitigate, manage, and – with any hope – defeat COVID-19, HIV patient advocacy has suffered greatly in the last decade. Arguably longer.
I’ve often mulled this conversation and the complex realities impacting the health of our aging advocate community. The truth is, many of the survivors of the AIDS Crisis never expected to live this long. All that work, all that fight, transformed into one of three paths; death, patient, or industry. Many people who took up the mantle of HIV patient advocacy or care delivery with extraordinary efficacy. To date, given the enormous obstacles of funding, access, stigma, and systemic biases, debatably, few public health campaigns have been as effective as those associated with the prevention, testing, and treatment of HIV. However, despite all of our successes and advocacy, according to the Centers for Disease Control and Prevention, progress in combatting the domestic HIV epidemic has stagnated over the last decade.
Central to this issue, few states have expanded their HIV funding in the last decade (kudos to Georgia’s HIV advocates for the recent influx of state funding to their State AIDS Drug Assistance Program), onerous rules on how to funds may be spent have left federal funds on the table, unused until usurped by political ideologues to put kids in cages, service providers are experiencing an extraordinary rate of burnout, competition in advocacy space has discouraged younger talent from staying in the field, or problematic policies or personalities in legacy institutions have pushed these young advocates to start their own agencies with little in the way of support – further compounding the field of competition.
Our ideas have aged as much as our leadership has. At the 30-thousand-foot view, this is one of many of the core reasons we’ve failed to advance in our fight against HIV.
In order to meaningfully advance HIV patient advocacy and thus work toward a more equitable regulatory and funding landscape, private funders and foundation partners need to re-examine “leadership development” initiatives and projects. Currently, these so-called “leadership development” programs are often short-lived, focus on recruiting a specific demographic audience, may share some story-telling skills, and maybe offer some networking among audience members. The idea being to further engage highly affected populations which may not otherwise fit traditional requirements in hiring and promotion screening. Often, these funds go to large entities which may lack specific expertise or experience with the intended demographic audience. Deliverables for these programs do not require hiring or connecting to employment opportunities for attendees, nor do they include requirements to advance leadership opportunities with existing staff or external recruitment from target demographics or affected community. These “leadership” programs lack any substantial applied benefit for affected communities, target demographics, or the patient advocacy landscape at large.
Internally, funders and providers required to engage community advisory boards often suffer the same stagnation. Community advisory boards maintain the same membership for years and years, with limited power of influence over industry activities, and zero incentive to seek new voices or experiences, including lack of compensation for time spent. To be clear, for our pharmaceutical manufacturing partners and government agency partners: community advisory boards should be disease state or demographic identity specific and not generalized for chronic illnesses or general populations in order to meaningfully affect the health and wellness of these communities, ensure equitable clinical trial and programmatic designs, and prioritize the needs of the impacted communities as those communities define them.
The argument goes “We need the institutional knowledge or connections. We can’t afford to lose the investment already made into these members.” And whole host of other reasons that amount to “that’s too hard.”
In order to move forward or even maintain effective advocacy, funders, both public and private, commercial industry and non-profit industry, need to re-work the deliverables associated with advancing leadership and recruiting and fostering advocacy expertise.
In short, it’s time to fund the retirement of aging leadership that never thought they’d live this long.
This is not a flippant suggestion, nor is it intended to provoke overnight changes that ultimately weaken the human infrastructure of advocacy. Rather, in order to meaningfully invest in the patient advocacy pipeline – and ultimately ensure shared interests of communities and industry and public health are met – “leadership” and “development” programs should aim to require, at least, the following, as appropriate:
Recruit candidates on the basis of advancing their personal careers and career goals, in alignment with the entity’s mission, as opposed to existing skill sets (job skills can be taught and indeed most are taught on the job)
Develop and implement a leadership change plan (ex. Year 1: identify candidates, Year 2: Mentor candidates in role, function, and networking. Year 3: Shadow candidate in performance of duties, critique and advise)
Review and update human resources policies including but not limited to education requirements for positions (ie. lived experience in lieu of formal education requirements), compensation relative to private industry competitors and best practices, and staff demographics relative to client demographics (these should be relatively on par to each other)
For conference style programs: require job placement or work search assistance until placement is found for the candidate
For community advisory boards: term limits, industry standard consultant compensation for board members, demographics reflecting that of affected communities, and member transition and mentoring programs aimed at recruiting
Part of how we’ve lost our way in advocacy is focusing on “cause” to drive interest and, frankly, cause does not keep the lights on. As non-profit industry moves to better understand that “cause over compensation” is robbing us of our best and brightest minds, so must our funders. We cannot recruit and retain the most creative generation of talent by racing to the cheapest contract.
President Biden is oft cited as saying “Don't tell me what you value, show me your budget, and I'll tell you what you value.” Though the sentiment has long existed and touted by others. And it misses the point of implementation.
Your integrity is spending your money in a way that actualizes your values.