Watch 03: July 2023
The HIV/HCV Co-Infection Watch is a project of the Community Access National Network (CANN) designed to research, monitor and report on HIV and Hepatitis C (HCV) co-infection in the United States. The July 2023 Watch includes timely updates herein. To read the project disclaimer and/or methodology, CLICK HERE.
1. FINDINGS
The following is a summary of the key findings for July 2023:
AIDS Drug Assistance Programs:
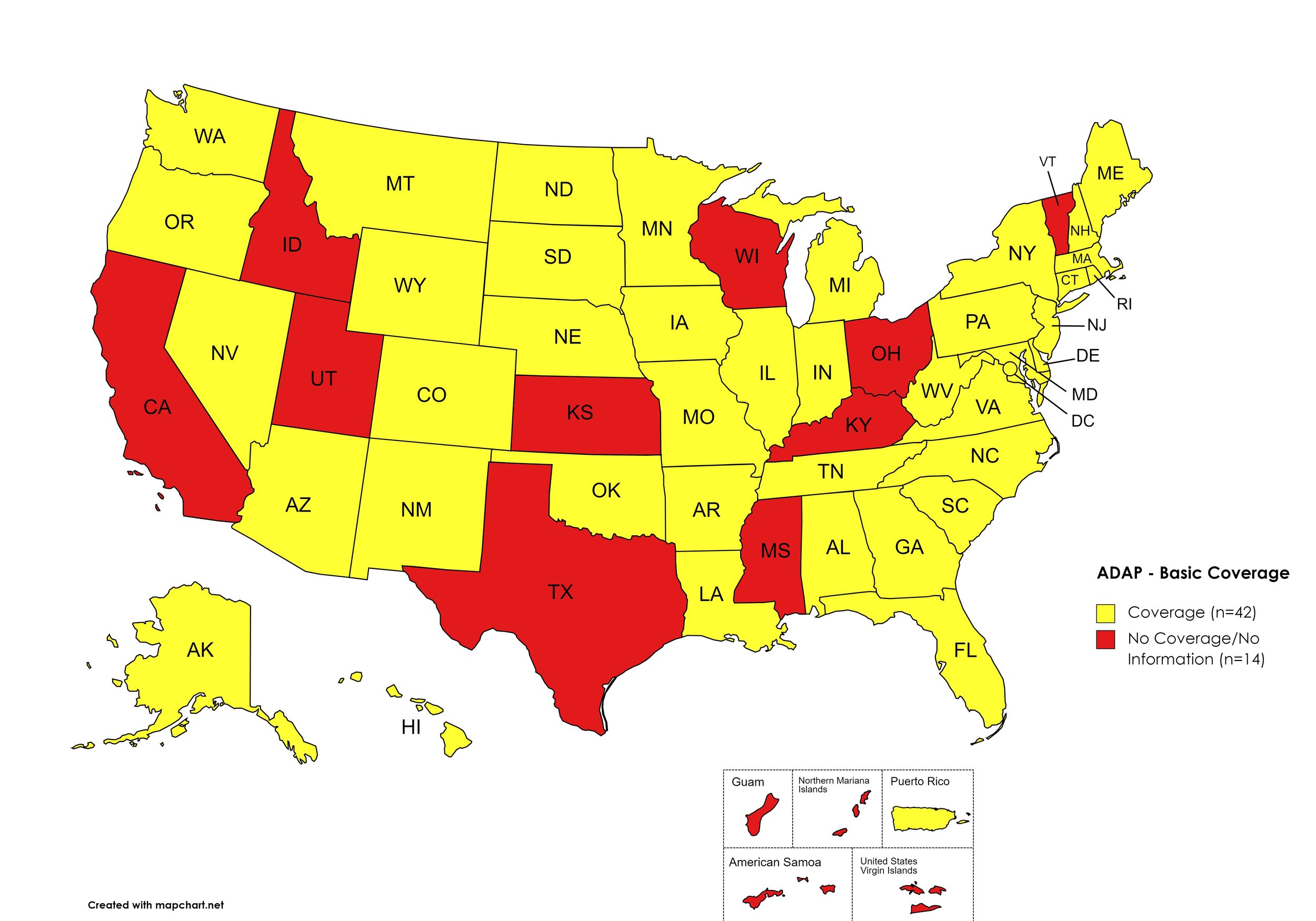
There are 56 State and Territorial AIDS Drug Assistance Programs (ADAPs) in the United States, 47 of which offer some form of coverage for Hepatitis C (HCV) treatment. Of those programs, 45 have expanded their HCV coverage to include the Direct-Acting Antiviral (DAA) regimens that serve as the current Standard of Care (SOC) for Hepatitis C treatment. Two (2) programs offer only Basic Coverage and 9 programs offer No Coverage. One (1) program covers only a single Direct-Acting Antiviral. Three (3) territories – American Samoa, Marshall Islands, and Northern Mariana Islands – are not accounted for in this data. A state-by-state Drug Formulary breakdown of coverage is included in the July 2023 Updates, with accompanying drug-specific maps in Figures 1 – 10.
Medicaid Programs:
There are 59 State and Territorial Medicaid programs in the United States, and data is represented for all fifty (50) states and the District of Columbia. As of October 01, 2016, all 50 states and the District of Columbia offer Expanded Coverage. A state-by-state PDL breakdown of coverage is included in the July 2023 Updates, with accompanying drug-specific maps in Figures 11 – 20.
Harm Reduction Programs:
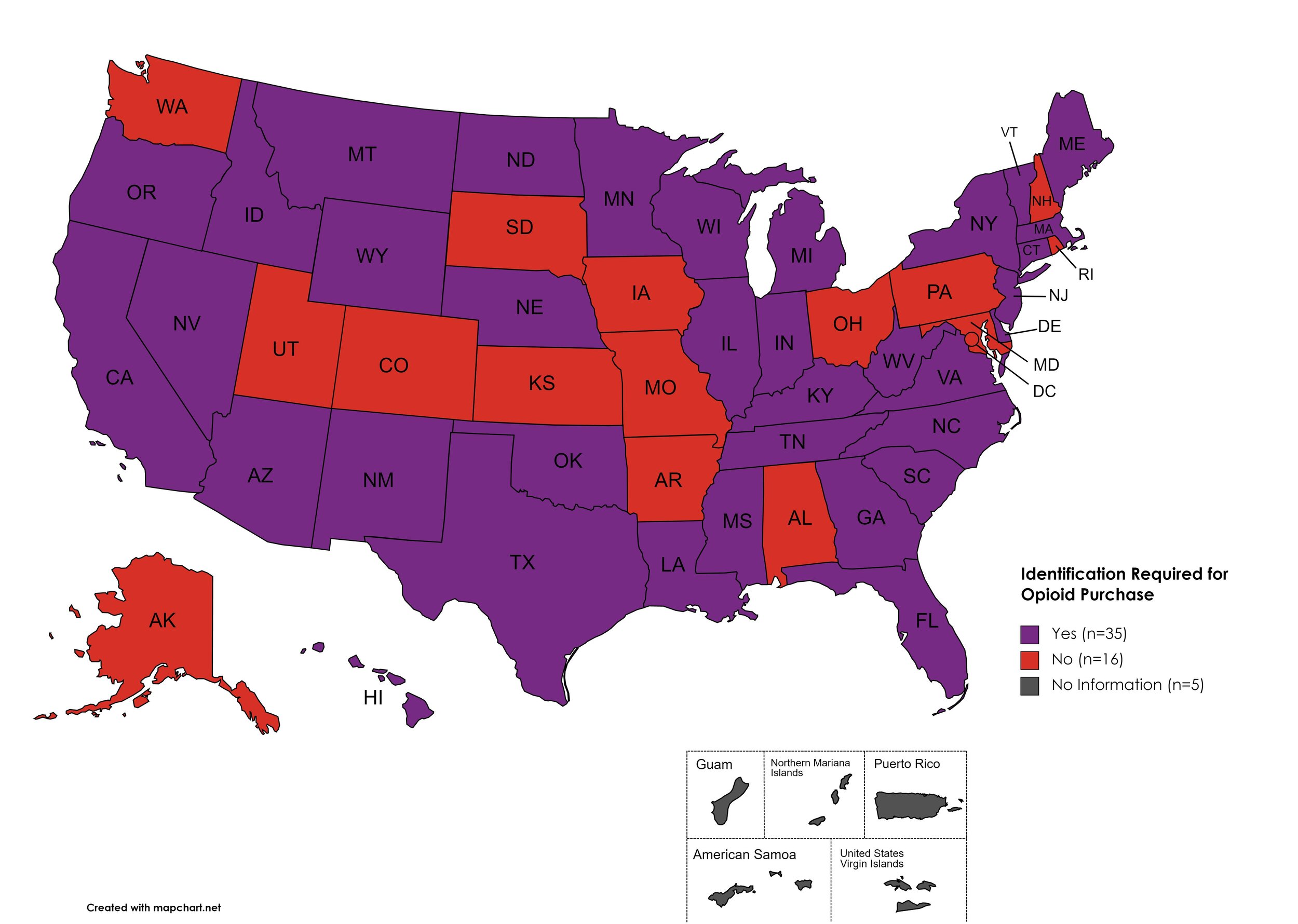
Every State and Territory in the United States currently provides funding for low-income people living with substance abuse issues to enter state-funded rehabilitation services (National Center for Biotechnology Information, n.d.). Forty-three (43) States, the District of Columbia and three (3) Territories currently have Syringe Services Programs (SSPs) in place, regardless of the legality. Fifty (50) States and the District of Columbia have expanded access to Naloxone to avert opioid drug overdoses. Fifty (50) States and the District of Columbia have Good Samaritan laws or statutes that provide some level of protection for those rendering emergency services during drug overdoses. Fifty (50) States, the District of Columbia, and Guam make reporting to Prescription Drug Monitoring Programs (PDMPs) mandatory, requiring physicians and/or pharmacists to report prescriptions written or filled to a state agency for monitoring. Fifty (50) States and the District of Columbia have Opioid-Specific Doctor Shopping Laws preventing patients from attempting to receive multiple prescriptions from numerous physicians, and/or from withholding information in order to receive prescriptions. Fifty (50) states and the District of Columbia mandate a Physical Exam Requirement in order for patients to receive a prescription for opioid drugs. Thirty-Five (35) states have in place an ID Requirement mandating that people filling opioid prescriptions present a state-issued ID prior to receiving their prescription. Forty-nine (49) states and the District of Columbia require prescribing physicians to attend mandatory and continuing opioid prescribing education sessions. Forty-seven (47) states and the District of Columbia have Medicaid doctor/pharmacy Lock-In programs that require patients to receive prescriptions from a single physician and/or fill prescriptions from a single pharmacy. A state-by-state program breakdown is included in the July 2023 Updates, with accompanying drug-specific maps in Figures 21-29.
2. AIDS DRUG ASSISTANCE PROGRAMS (ADAPs) & HCV THERAPIES
Of the 56 respective State and Territorial ADAPs, only 8 (KS, KY, OH, UT, VT, GU, PW, VI) do not offer any coverage for HCV drug therapies. States whose formularies are not available on the state-run website have been checked against the most recent National Alliance of State and Territorial AIDS Directors (NASTAD) formulary database (last updated January 1, 2023). The data presented are current as of July 9, 2023.
July 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AL, AK, AZ, AR, CO, CT, DE, FL, GA, HI, IL, IN, IA, LA, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OR, PA, RI, SC, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Basic HCV Medications Coverage: CA, ID, KS, KY, MS, OH, TX, UT, VT
Territories with Basic HCV Medications Coverage: P.R.
Figure 1. July 2023 ADAP Coverage - Basic
Map Key: Yellow = Basic Coverage; Red = No Basic Coverage/No Information regarding Basic Coverage
Sovaldi
States with Sovaldi Coverage: AZ, CA, CO, GA, HI, IL, IN, IA, LA, ME, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OK, OR, PA, SD, VA, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AR, CT, DE, FL, ID, KS, KY, MI, MS, MO, MT, NY, NC, OH, RI, SC, TN, TX, UT, VT, WV
Territories with Sovaldi Coverage: P.R.
Figure 2. July 2023 ADAP Coverage - Sovaldi
Map Key: Yellow = Sovaldi Coverage; Red = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, VA, WA, WI, WY, D.C.
States without Harvoni Coverage: AL, AK, KS, KY, MO, MT, NY, OH, RI, SC, TX, UT, VT, WV
Territories with Harvoni Coverage: P.R.
Figure 3. July 2023 ADAP Coverage - Harvoni
Map Key: Yellow = Harvoni Coverage; Red = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AZ, AR, CA, CO, FL, GA, HI, IL, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, VA, WA, WV, WI, WY, D.C.
States without Zepatier Coverage: AK, CT, DE, ID, IN, KS, KY, MO, MT, OH, OK, RI, SC, TN, TX, UT, VT
Territories with Zepatier Coverage: P.R.
Figure 4. July 2023 ADAP Coverage - Zepatier
Map Key: Yellow = Zepatier Coverage; Red = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, NE, NY, NV, NH, NJ, NM, ND, OR, PA, SD, TN, VA, WA, WI, WY
States without Epclusa Coverage: AL, AK, DE, KS, KY, MT, NC, OH, OK, RI, SC, TX, UT, VT, WV, D.C.
Territories with Epclusa Coverage: P.R.
Figure 5. July 2023 ADAP Coverage - Epclusa
Map Key: Yellow = Epclusa Coverage; Red = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: CA, CT, FL, HI, ID, IL, IN, IA, LA, MD, MA, MN, NE, NV, NH, NJ, NM, ND, OR, SD, TN, WA, WY
States without Vosevi Coverage: AL, AK, AZ, AR, CO, DE, GA, KS, KY, ME, MI, MS, MO, MT, NY, NC, OH, OK, PA, RI, SC, TX, UT, VT, VA, WV, WI, D.C.
Territories with Vosevi Coverage: P.R.
Figure 6. July 2023 ADAP Coverage - Vosevi
Map Key: Yellow = Vosevi Coverage; Red = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OR, PA, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Mavyret Coverage: AK, DE, KS, KY, OH, OK, RI, SC, TX, UT, VT
Territories with Mavyret Coverage: P.R.
Figure 7. July 2023 ADAP Coverage - Mavyret
Map Key: Yellow = Mavyret Coverage; Red = No Mavyret Coverage/No Information regarding Mavyret Coverage
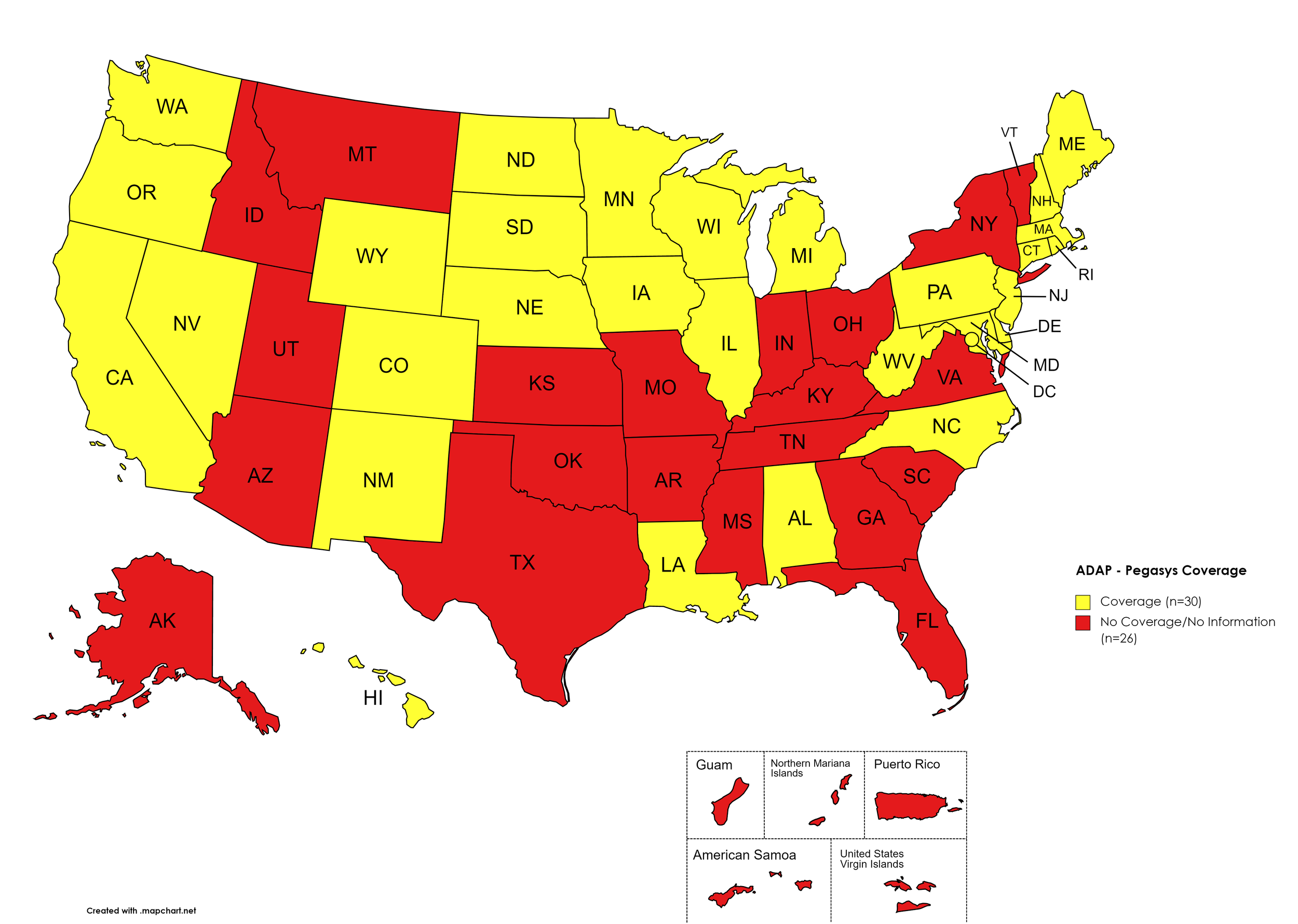
Pegasys
States with Pegasys Coverage: AL, CA, CO, CT, DE, HI, IL, IA, LA, ME, MD, MA, MI, MN, NE, NV, NH, NJ, NM, NC, ND, OR, PA, RI, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Pegasys Coverage: AK, AZ, AR, FL, GA, ID, IN, KS, KY, MS, MO, MT, NY, OH, OK, SC, TN, TX, UT, VT, VA
Territories with Pegasys Coverage: None/Unknown
Figure 8. July 2023 ADAP Coverage - Pegasys
Map Key: Yellow = Pegasys Coverage; Red = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AZ, AR, CA, CO, CT, FL, IL, IA, ME, MD, MA, MN, MS, NE, NV, NH, NJ, NM, NC, ND, OK, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Harvoni (generic)Coverage: AL, AK, DE, GA, HI, ID, IN, KS, KY, LA, MI, MO, MT, NY, OH, RI, SC, TX, UT, VT, VA, WV
Territories with Harvoni (generic) Coverage: P.R.
Figure 9. July 2023 ADAP Coverage - Harvoni (Generic)
Map Key: Yellow = Harvoni (Generic) Coverage; Red = No Harvoni (Generic) Coverage/No Information regarding Harvoni (Generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AZ, AR, CA, CO, CT, FL, IL, IN, IA, ME, MD, MA, MN, MS, MO, NE, NV, NH, NJ, NM, ND, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Epclusa (generic) Coverage: AL, AK, DE, GA, HI, ID, KS, KY, LA, MI, MT, NY, NC, OH, OK, RI, SC, TX, UT, VT, VA, WV
Territories with Epclusa (generic) Coverage: P.R.
Figure 10. July 2023 ADAP Coverage - Epclusa (generic)
Map Key: Yellow = Epclusa (generic) Coverage; Red = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
July 2023 Notes:
States with Open Formularies: IL, IA, MA, MN, NE, NH, NJ, NM, ND, OH, OR, WA, WY
N.B. – Although Ohio is listed by NASTAD as having an open formulary, both NASTAD’s ADAP Formulary Database and Ohio’s ADAP website indicates that the state does not offer any treatment for HCV.
N.B. – Although North Dakota has adopted an open formulary, they provide only co-pay and deductible assistance for HCV medications.
N.B. – Wyoming's ADAP Open Formulary document, the following disclaimer related to HCV is made: Hepatitis C treatment medications (i.e. Harvoni, Sovaldi, Ribavirin, Zepatier, Epclusa) must be prior authorized. To be eligible, clients must have applied for prior authorization from their insurance plan and the WY ADAP Hepatitis C Treatment checklist must be completed and signed by the provider and client.
Colorado offers five coverage options – Standard ADAP, HIV Medical Assistance Program (HMAP), Bridging the Gap Colorado (BTGC), HIV Insurance Assistance Program (HIAP), and Supplemental Wrap Around Program (SWAP). ‘Yes’ indications in Figure 1. for Colorado denote that at least one of these programs offers coverage for each respective drug. The Standard ADAP Formulary covers medications only if funds are available to do so.
Louisiana’s ADAP (Louisiana Health Access Program – LA HAP) offers two coverage options – Uninsured (Louisiana Drug Assistance Program – L-DAP) and Insured (Health Insurance Program – HIP). HIP pays for the cost of treatment only if the client’s primary insurance covers the drug under its formulary.
Georgia’s ADAP notes the following: “Georgia ADAP Hepatitis C Program is currently on HOLD until future funding is available. Please utilize Patient Assistance Programs (PAP’s) for Hepatitis C medications.”
After initial publication of this report, on August 11th, the Georgia Department of Public Health issued a notice to Ryan White Part B District Coordinators, reading, in part, “Effective 8/14/2023, care providers will have the ability to order Hepatitis C medications for their eligible ADAP patients without the need for Prior Approval.”
Texas ADAP maintains no HCV coverage, despite a brief period of covering DAAs in 2022.
California’s ADAP pharmacy benefit manager has recently removed coverage of ribavirin products for the treatment of HCV.
3. MEDICAID PROGRAMS & HCV THERAPIES
All 50 states and the District of Columbia continue to offer some form of HCV coverage. All 50 states and the District of Columbia have expanded their Preferred Drug Lists to include at least one HCV Direct Acting Agent (DAA).
July 2023 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AZ, AK, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Basic HCV Medications Coverage: AL, ID, KS, MO, OK, SC, VA, WY
Figure 11. July 2023 Medicaid Coverage - Basic HCV Medications
Map Key: Blue = Basic HCV Medication Coverage; Yellow = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Sovaldi Coverage: AL, AK, AZ, CT, FL, IA, NH, NM, OK, OR, SC, VA, WV, WY.
Figure 12. July 2023 Medicaid Coverage - Sovaldi
Map Key: Blue = Sovaldi Coverage; Yellow = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni Coverage: AK, AZ, CT, FL, IA, NM, OK, OR, SC, VA, WY.
Figure 13. July 2023 Medicaid Coverage - Harvoni
Map Key: Blue = Harvoni Coverage; Yellow = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Zepatier Coverage: AK, AZ, CT, FL, IA, NH, NM, OK, OR, SC, VA, WV, WY.
Figure 14. July 2023 Medicaid Coverage - Zepatier
Map Key: Blue = Zepatier Coverage; Yellow = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AL, AR, CA, CO, DE, GA, HI, IL, IN, KS, KY, LA, MA, ME, MI, MN, MS, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Epclusa Coverage: AK, AZ, CT, FL, ID, IA, MD, NE, OK, SC, VA, WY.
Figure 15. July 2023 Medicaid Coverage - Epclusa
Map Key: Blue = Epclusa Coverage; Yellow = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SC, SD, TN, TX, UT, VT, WA, WI, D.C.
States without Vosevi Coverage: AL, AK, AZ, NM, OK, OR, VA, WV, WY.
Figure 16. July 2023 Medicaid Coverage - Vosevi
Map Key: Blue = Vosevi Coverage; Yellow = No Vosevi Coverage/No Information regarding Vosevi Coverage
Mavyret
States with Mavyret Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Figure 17. July 2023 Medicaid Coverage - Mavyret
Map Key: Blue = Mavyret Coverage; Yellow = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AK, AZ, CA, CT DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, OH, OR, PA, RI, SD, TN, TX, VT, WA, WV, WI, D.C.
States without Pegasys Coverage: AL, AR, CO, ID, KS, MO, ND, OK, SC, UT, VA, WY
Figure 18. July 2023 Medicaid Coverage - Pegasys
Map Key: Blue = Pegasys Coverage; Yellow = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AL, AR, CA, CO, DE, GA, HI, ID, IL, IN, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NY, NC, ND, OH, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni (generic) Coverage: AK, AZ, CT, FL, IA, KS, NM, OK, OR, SC, VA, WY
Figure 19. July 2023 Medicaid Coverage - Harvoni (generic)
Map Key: Blue = Harvoni (generic) Coverage; Yellow = No Harvoni (generic) Coverage/No Information regarding Harvoni (generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AK, AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Epclusa (generic) Coverage: ID, OK
Figure 20. July 2023 Medicaid Coverage - Epclusa (generic)
Map Key: Blue = Epclusa (generic) Coverage; Yellow = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
July 2023 Notes:
The follow states’ Medicaid programs offer multiple coverage plans for their respective Medicaid clients. The plan highlighted in bold typeface represents the most comprehensive plan with the most drugs covered in the respective state:
Hawaii – (1.) Advantage Plus; (2.) QUEST Integration
New Jersey – (1.) Aetna; (2.) AmeriGroup NJ; (3.) Horizon NJ Health; (4.) UnitedHealthcare of New Jersey; (5.) WellCare
New Mexico – (1.) BlueCross BlueShield of New Mexico; (2.) Presbyterian Centennial Care; (3) Western Sky Community Care
Kentucky has a Unified Medicaid Formulary
Louisiana has a Unified Medicaid Formulary
Ohio – Ohio has a Unified Medicaid Formulary that applies to all MCOs
Oregon’s Medicaid program removed coverage of Sovaldi.
Texas’ Medicaid DPL has not changed, however, the program site notes that Mvyret is now the only preferred DAA, which will no longer require a prior authorization.
New Hampshire’s Medicaid program has removed Zepatier and Sovaldi from the formulary.
Oklahoma’s Medicaid program has reduced coverage to only Mavyret.
Wyoming’s Medicaid program has reduced coverage to only ribavirin products, Mavyret, and Epclusa (GENERIC).
No data is has been made available by the Medicaid programs in the U.S. Territories.
*Medicaid coverage excludes patients from most drug manufacturer patient assistance programs (PAPs)
4. VETERANS PROGRAMS & HCV THERAPIES
The Veteran's Administration (VA) currently offers coverage for all HCV drugs. This is according to the most recent VA National Formulary, dated May 2021 (U.S. Dept. of V.A., 2021a). The VA Treatment Considerations and Choice of Regimen for HCV-Mono-Infected and HIV/HCV Co-Infected Patients, dated March 2021 (U.S. Dept. of V.A., 2021b) lists the following therapies as preferred treatments:
Abbreviations:
- CTP – Child-Turcotte-Pugh (score used to assess severity of cirrhosis)
- IU/mL – International Units Per Milliliter
- PEG-IFN/IFN – Peginterferon/Interferon
- RAS – Resistance-associated substitutions
Genotype 1:
Treatment-naïve without or with cirrhosis (CTP A):
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens:
Zepatier: 1 tablet orally daily for 12 weeks if GT1a without baseline NS5A RAS or GT1b
Harvoni: 1 tablet orally daily
If HCV-noninfected, non-cirrhotic, and HCV RNA baseline <6 million IU/mL: 8 weeks
If cirrhotic, baseline HCV RNA ≥6 million IU/mL, HIV/HCV-co-infected, or African American: 12 weeks
Consider adding ribavirin in CTP A patients
Treatment-naïve with decompensated cirrhosis (CTP B or C):
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Epclusa: 1 tablet orally daily + ribavirin (1000 mg/day - <75kg – or 1,200 mg daily - ≥75kg – orally daily in 2 divided doses with food) for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb).
Treatment-experienced (NS5A- and SOF-naïve [e.g., failed PEG-IFN/RBV ± NS3/4A PI]) without or with cirrhosis (CTP A)
Pangenotypic regimens:
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If NS3/4A PI + PEG-IFN/RBV-experienced: 12 weeks
Vosevi: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks if GT1b, or if failed only PEG-IFN/RBV and GT1a without baseline NS5A RAS
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-naïve and SOF-experienced) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV + Sovaldi-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If Olysio + Sovaldi-experienced: 12 weeks
Epclusa: 1 tablet orally daily for 12 weeks if GT1b
Vosevi: 1 tablet orally daily with food for 12 weeks if GT1a
Treatment-experienced (prior NS5A-containing regimen) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks if failed only an NS5A inhibitor without NS3/4A PI (e.g., Harvoni)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or C)
Epclusa: 1 tablet orally daily + RBV; start at lower RBV doses as clinically indicated (e.g., baseline Hgb);
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
Genotype 2:
Treatment-naïve or treatment-experienced (PEG-IFN/IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; 12 weeks if CTP A and treatment-experienced or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-naïve or treatment-experienced patients with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 3:
Treatment-naïve without cirrhosis or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks if cirrhotic or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
If CTP A, test for NS5A RAS
Add ribavirin if Y93H RAS present
Treatment-experienced (PEG-IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
If CTP A, consider adding ribavirin (no supporting data)
Treatment-naïve or treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 4:
Treatment-naïve without or with cirrhosis (CTP A)
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-naïve with decompensated cirrhosis (CTP B or C)
Pangenotypic regimen
Epclusa: 1 tablet orally daily + RBV for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Non-pangenotypic regimen:
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Treatment-experienced (Sovaldi-experienced and NS5A-naïve) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks if NS3/4A PI-naïve without cirrhosis, and 12 weeks if NS3/4A PI-experienced or CTP A
Epclusa: 1 tablet orally daily + ribavirin for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
5. PATIENT ASSISTANCE PROGRAMS
The drug manufacturers and various national nonprofit organizations offer a variation of patient assistance programs (PAPs) to assist patients in accessing treatments. They include:
Support Path (Gilead Sciences):
Financial Assistance
Provides Co-Pay Coupons for Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), and Vosevi
Co-Pay Coupons cover out-of-pocket costs up to 25% of the catalog price of a 12-week regimen (3 bottles/packages) of Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), or Vosevi
Excludes patients enrolled in Medicare Part D or Medicaid
Insurance Support
Researches and verifies patient’s benefits, and gives information they need about coverage options and policies
Explain Prior Authorization process and works with HCV Specialist’s office so they can submit PA forms to a patient’s insurance company
May be able to provide assistance with appeals process
Website: http://www.mysupportpath.com/
AbbVie Mavyret Co-Pay Savings Card:
Financial Assistance
Patient may be eligible to pay as little as $5
Excludes patients enrolled in Medicare Part D, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs)
NeedyMeds:
NeedyMeds Drug Discount Card
Designed to lower cost of prescription medications by up to 80% at participating pharmacies
Price finder tool for the drug discount card
No eligibility requirements
CANNOT be used in combination with government healthcare programs, but CAN be used IN PLACE of program
CANNOT be combined with other offers
Website: http://ow.ly/fEJo309cJ7Z
The Assistance Fund:
Status: WAITLISTED
Requires provider referral
Copay assistance
Eligibility Criteria:
US citizen or permanent resident
Diagnosed with the disease for which you are applying
Prescribed an FDA-approved treatment for the disease
Have prescription coverage for the prescribed treatment
Meet financial eligibility criteria based upon household income and size
Patient Advocate Foundation Co-Pay Relief:
Status: CLOSED
Maximum award of $15,000
Eligibility Requirements:
Patient must be insured, and insurance must cover prescribed medication
Confirmed HCV diagnosis
Reside and receive treatment in the U.S.
Income falls below 400% of FPL with consideration of the Cost of Living Index (COLI) and the number in the household
Patient Access Network (PAN) Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $5,600
Patients may apply for additional assistance during their eligibility period, subject to availability of funding
Eligibility Requirements:
Must be getting treatment for HCV
Have insurance that covers prescribed HCV medication
Medication must be listed on PAN’s list of covered medications: https://www.panfoundation.org/index.php/en/patients/medications-covered
Income falls below 500% of FPL
Residing and receiving treatment in the U.S. (citizenship NOT required)
Website: https://www.panfoundation.org/index.php/en/patients/assistance-programs/hepatitis-c
HealthWell Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $30,000
Minimum Co-Pay Reimbursement Amount: None
Minimum Premium Reimbursement Amount: None
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Income falls below 500% of FPL
Receiving treatment in the U.S.
Website: https://www.healthwellfoundation.org/fund/hepatitis-c/
6. HARM REDUCTION PROGRAMS
Harm Reduction, as it relates to opioid abuse and HCV, are measures designed to serve as preventive or monitoring efforts in combating opioid prescription drug and heroin abuse, and as an effect, helping to prevent the spread of HCV and HIV. The Co-Infection Watch covers the following measures: Syringe Exchange, Expanded Naloxone Access, Good Samaritan Laws, Mandatory PDMP Reporting, Doctor Shopping Laws, Physical Exam Requirements, ID Requirements for Purchase, Required or Recommended Prescriber Education, and Lock-In Programs (Editor’s Note: Program descriptions provided herein).
July 2023 Updates:
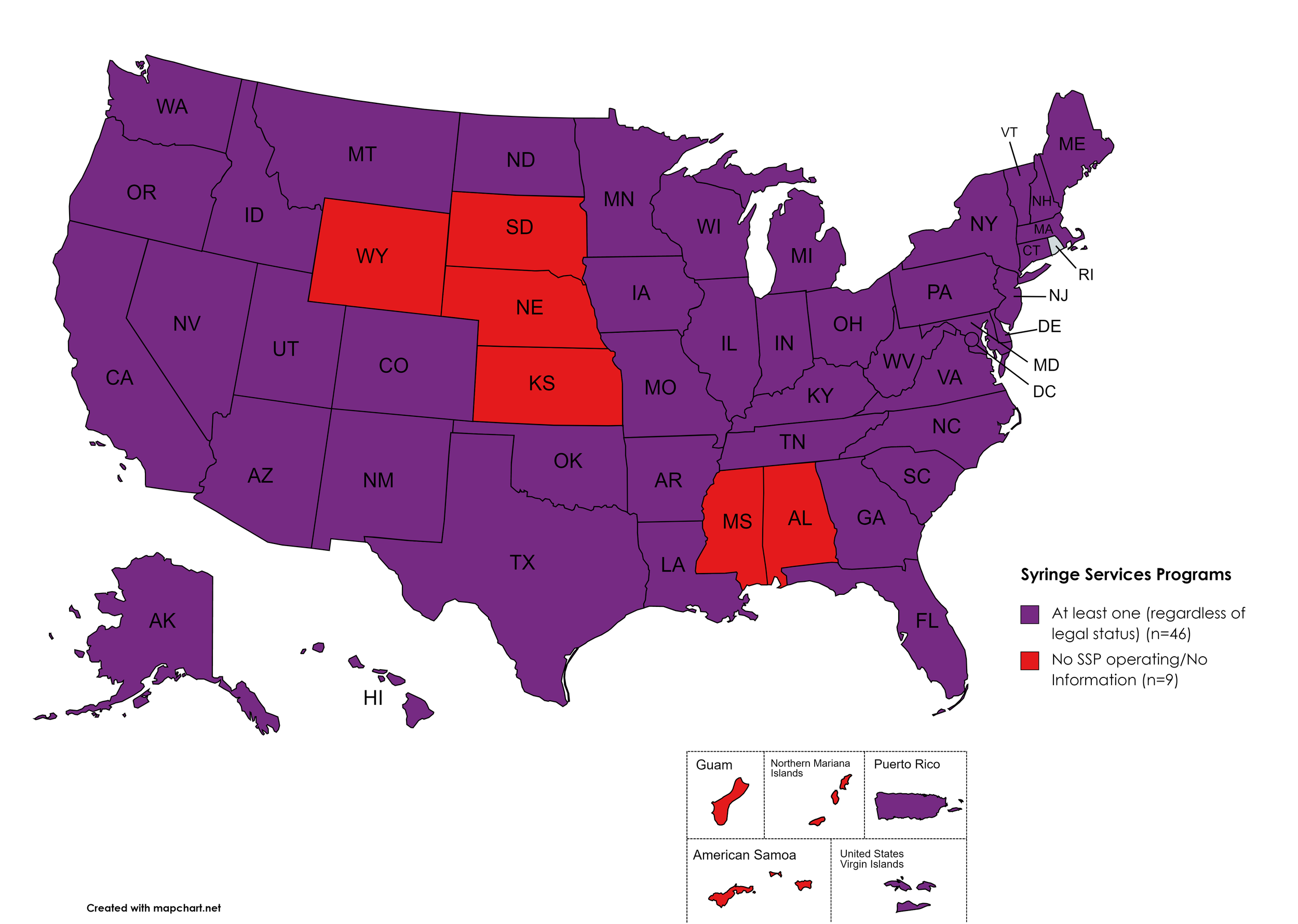
Syringe Exchange
Syringe Services Programs (SSPs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones. (N.b. – states listed as "at least one SSP…” indicate only that a Syringe Services Program (SSP) exists within the state, regardless of the legality of SSPs under state law).
States with Syringe Exchange: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, D.C.
States without Syringe Exchange: AL, KS, MS, NE, SD, WY
Territories with Syringe Exchange: Puerto Rico, U.S. Virgin Islands
Figure 21. July 2023 Syringe Exchange Coverage
Map Key: Purple = Syringe Exchange(s); Red = No Syringe Exchange(s); Grey = No Information
Expanded Naloxone
Naloxone is a drug used to counteract the effects of opioid overdoses. Expanded Access refers to one of more of the following conditions: Naloxone purchase without a prescription; availability to schools, hospitals, and emergency response units for use in the event of an overdose.
States with Expanded Naloxone: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Expanded Naloxone: None
Territories with Expanded Naloxone: Unknown
Figure 22. July 2023 Expanded Naloxone Coverage
Map Key: Purple = Expanded Naloxone; Red = Restricted Naloxone; Gray = No Information
Good Samaritan Laws
Good Samaritan Laws are laws that are designed to protect emergency services personnel, public or private employees, and/or citizens from being held legally liable for any negative healthcare outcomes as a result of providing "reasonable measures" of emergent care.
States with Samaritan Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Samaritan Laws: None
Territories with Samaritan Laws: Unknown
Figure 23. July 2023 Good Samaritan Laws Coverage
Map Key: Purple = Good Samaritan Laws; Red = No Good Samaritan Laws; Gray: No Information
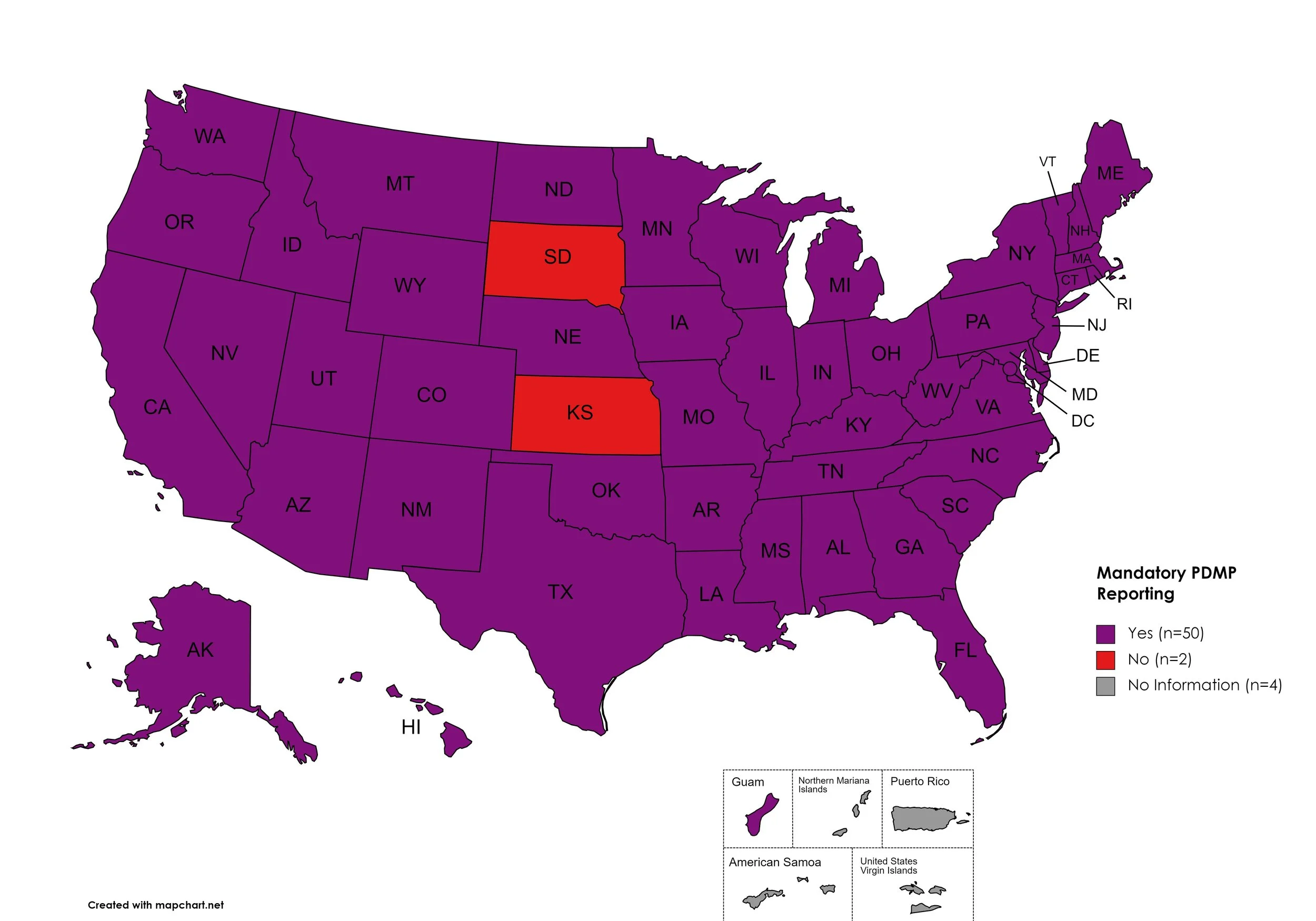
Mandatory PDMP Reporting
Prescription Drug Monitoring Programs (PDMPs) are programs established by state and/or federal law that requires prescribing physicians and/or the fulfilling pharmacies to register with a state agency. While every state has established required enrollment in a PDMP for prescribers, not all states mandate dispensing entities to enroll. Similarly, prescription reporting to a state agency may include one or more of the following data points: Patient Names; Specific Drug(s) Prescribed; Prescription Dosage; Date; Time; Form of State-Issued ID.
States with Mandatory PDMP Reporting: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Mandatory PDMP Reporting: SD, KS
Territories with Mandatory PDMP Reporting: Guam
Figure 24. July 2023 Mandatory Prescription Drug Monitoring Program Coverage
Map Key: Purple = Mandatory PDMP; Red = No Mandatory PDMP; Gray = No Information
Doctor Shopping Laws
Doctor Shopping Laws are those laws designed to prevent patients from seeking one or more of the same prescription from multiple doctors through the use of subterfuge, falsifying identity, or any other deceptive means. While federal law prohibits Doctor Shopping, most states also include provisions that prohibit patients from seeking a new prescription if another physician has denied a similar prescription within a certain period of time.
States with Doctor Shopping Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Doctor Shopping Laws: None
Territories with Doctor Shopping Laws: None
Figure 25. July 2023 Doctor Shopping Laws Coverage
Map Key: Purple = Doctor Shopping Laws; Red = No Doctor Shopping Laws; Grey = No Information
Physical Exam Required
Physical Exam Requirements are those that mandate that the prescribing physician perform a physical examination on a patient before providing a prescription for a controlled substance to determine if the prescription is medically necessary. Many states have expanded their definitions of “physical exam” to include a physical exam via telehealth when such an exam is similarly significant and sufficient as being seen in-person.
States with Physical Exam Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, MD, MA, ME, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, PA, RI, SC, SD, TN, TX, UT, VA, VT, WA, WV, WI, WY, D.C.
States without Physical Exam Required: SD
Territories with Physical Exam Required: None
Figure 26. July 2023 Physical Exam Required Coverage
Map Key: Purple = Physical Exam Required; Red: No Physical Exam Required; Grey = No Information
I.D. Required for Purchase of Opioid Prescription
Federal law requires anyone purchase a controlled substance to provide a state-issued identification (“I.D.”) in order to fill the prescription. Mandatory ID requirements go further and require that this information be recorded and stored in an effort to prevent the same patient from obtaining multiple or repeated prescriptions in a given period of time.
States with I.D. Required: AZ, CA, CT, DE, FL, GA, HI, ID, IL, IN, KY, LA, ME, MA, MI, MS, MN, MT, NE, NV, NJ, NM, NY, NC, ND, OK, OR, SC, TN, TX, VT, VA, WV, WI, WY
States without I.D. Required: AL, AK, AR, CO, IA, KS, MD, MO, NH, OH, PA, RI, SD, UT, WA, D.C.
Territories with I.D. Required: Unknown
Figure 27. July 2023 I.D. Required Coverage
Map Key: Purple = I.D. Required; Red = No I.D. Required; Gray = No Information
Prescriber Education Required
States that require/do not require that prescribing physicians undergo special training in addition to or as part of their initial education to become prescribers related to safer controlled substance and/or pain management prescribing and utilization practices.
States with Prescriber Education Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MO, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Prescriber Education Required: MT, SD
Territories with Prescriber Education Required: Unknown
Figure 28. July 2023 Prescriber Education Required Coverage
Map Key: Purple = Prescriber Ed Required; Red = No Prescriber Ed Required; Gray = No Information
Medicaid Lock-In Program
Lock-In Programs are laws requiring that patients either receive prescriptions from only one physician and/or fill prescriptions from only one pharmacy.
States with Medicaid Lock-In Program: AL, AK, AZ, AR, CA, CO, CT, DE, GA, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Medicaid Lock-In Program: FL, HI, SD
Territories with Medicaid Lock-In Program: Unknown
Figure 29. July 2023 Medicaid Lock-In Coverage
Map Key: Purple = Medicaid Lock-In; Red = No Medicaid Lock-In; Gray = No Information
July 2023 Notes:
Metric definition for Mandatory PDMP Reporting section has been updated for clarity, the new map reflects the following:
Mandated prescriber and/or dispenser reporting only and do not reflect mandatory enrollment of prescribers or dispensers.
Most states do not require enrollment or of or reporting from both prescribers and dispensers; rather, most states with requirements require one or the other.
Many states have specific exceptions for reporting.
This adjustment clarifies that SD and KS are the only two states without required PDMP reporting by a prescriber or dispenser.
This adjustment clarifies that MT does require PDMP reporting by a prescriber or dispenser.
Metric definition for Physician Education has been updated to exclude “recommended” and only reflect those states which have laws or licensing board requirements of initial and/or continuing education for prescribers with regard to pain management and/or the prescription of controlled substances.
Some states have general requirements regarding “controlled substances”, some states are explicit with regard to category of controlled substance or type of controlled substance (ie. “opioids”).
This adjustment clarifies that MT and SD are the only states that do not require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
This adjustment clarifies that KS, MO, and ND do require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
Metric definition for Physical Exam has been updated for clarity regarding telehealth.
Many states have integrated statutory language requiring an exam in order to prescribe medication or otherwise establish an authentic patient-provider relationship to include telemedicine technologies. These technologies must be similarly sufficient to meet the standards of care and facilitate a similarly situated experience as to an in-person exam.
This adjustment clarifies that SD is now the only state which does not require a physical exam in-person or via sufficiently similar telehealth methods.
This adjustment means KS, MT, OR, and WI laws capture an exam requirement in order for a provider to establish an authentic patient-provider relationship and prescribe medications, including controlled substances.
CANN is no longer able to independently verify the existence of an SSP in Kansas. KS state laws prohibit SSPs and syringes are included in the state’s drug paraphernalia law.
The DEA’s COVID-19-based waiver of in-person exams is expected to expire in November of 2023. A final rule on this change has not yet been published as of the time of this report. The DEA’s proposed rule was met with sufficient enough advocate, patient, and provider objection that it will not be taken up and a new proposed rule is being drafted as of the time of this writing.
7. COVID-19 IMPACT ON HIV & HCV
The Community Access National Network’s blog began 2021 by assessing COVID-19’s impact on HIV, HCV, and Substance-Use Disorder. We will continue to monitor developments in light of the ongoing COVID-19 pandemic and its impacts on public health until January 2024. Beginning in January 2024, issues related to public health policy stemming from COVID-19 impacts will be included in the “Latest News” section of the Watch.
Additional Resources and Relevant Issues:
End of COVID-19 PHE - The COVID-19 Public Health Emergency officially ended at the end of the day on May 11th, 2023. The U.S. Department of Health and Human Services (HHS) fact sheet provides important updates regarding pandemic program continuity, wind-down, and cessation.
Medicaid Unwinding Tracker - KFF’s Medicaid Unwinding Tracker provides timely updates monitoring state enrollment and unwinding data and national enrollment data. As of the time of this writing, more than 3 million people have been disenrolled from Medicaid programs based on data provided by just 35 states and the District of Columbia. Variation in disenrollment ranges from 82% in Texas and 10% in Michigan. Overall, 74% of disenrollments are due to procedural reasons, rather than qualification reasons, with children accounting for about 31% of those persons disenrolled.
Early Data Shows that Many Recipients are Losing Coverage for Procedural Reasons - In May, The New York Times covered the issue of procedural disenrollments during the Medicaid unwinding period. Since then, the number of affected persons has grown at a startling rate. Most disenrollments are not the result of any lack of qualification on the recipients’ part but because of factors beyond their control. A procedural disenrollment is one which occurs not because of any qualification change for a recipient, but because a renewal process was not completed. This has left questions as to the sufficiency of state outreach efforts, which are mandated.
Biden Administration Concerned About Medicaid Disenrollments - Officials from the Centers for Medicare and Medicaid Services (CMS) held a call with reporters on Wednesday, July 19th, to discuss concerns as to the high rate and speed in which Medicaid recipients are being disenrolled after continuous coverage requirements related to the COVID-19 public health emergency ended. Officials shared they expected the unwinding to be a hectic time but were working with states to understand the issues. While the federal government told states to “take their time” and gave about a year for states to appropriately manage their Medicaid rolls in accordance with regular qualification and procedures, some states passed legislation mandating a faster pace and others have not sufficiently budget enough staff to navigate the unwinding.
CMS Announces Actions Against States Over Medicaid Redeterminations - On July 19th, Centers for Medicare and Medicaid Services (CMS) disclosed certain actions would be taken to halt Medicaid disenrollments in six states. CMS declined to name the states - due to the speed in which recipients were being kicked out of coverage. Qualification disenrollments may continue but procedural disenrollments must stop and those affected by procedural disenrollments must be reinstated into the program. CMS is also monitoring another 12 states to determine if they’re in violation of general Medicaid regulations and those specific to the unwinding process. Under penalty of losing federal matching dollars, which fund a large majority of the program, states are being encouraged to exercise waivers and make efforts to make navigating eligibility easier on potential recipients. Of particular concern, one of the states being ordered to temporarily cease procedural disenrollments did not provide some enrollees with renewal forms and another didn’t implement auto-renewal mitigation strategies. States are required to make significant effort to reach recipients, though what constitutes “significant” is not well-defined.
CDC Facing Major Funding Cuts, with Direct Impact on State and Local Health Departments - During the federal budget negotiation process, legislators are proposing drastic cuts to the Centers for Disease Control and Prevention (CDC). These cuts could prove catastrophic for state and local health departments dependent upon grants to fund their work in offering childhood vaccines, stemming the tide of sexually transmitted diseases, combatting maternal mortality, offering smoking cessation education, and more. Due to political backlash from the COVID-19 pandemic, some legislators animus toward the CDC is threatening these vital programs. Some states have more vacancies in their health departments than staff. The public health safety net is at risk.
CDC Announces Program for Free COVID-19 Vaccines to Uninsured and Underinsured Adults in Fall of 2023 - (The Centers for Disease Control and Prevention (CDC) will begin offering a program called “Bridge Access” this fall and through December 2024 to provide no-cost COVID-19 vaccines to uninsured and underinsured adults. Previously, under the public health emergency, COVID-19 vaccines were provided at no-cost because of appropriated funding dedicated specifically to provide those vaccines during the pandemic. As the unwinding of the public health emergency continues, the Bridge Access Programs seeks to provide a temporary safety net for uninsured or underinsured persons.
8. LATEST NEWS
HIV Infections Drop, but Racial Gaps Remain - In May, Politico’s Alice Ollstein covered the issue of HIV disparities and pandemic disruptions to care and prevention in the midst of debt ceiling limit negotiations. HIV diagnoses dropped about 12 percent from 2017 to 2021, though young people between the ages of 13 and 24 made up the bulk of reductions, with a 34% reduced rate of new diagnoses. The article focuses on PrEP access as being uneven or at least underutilized as a factor driving the disparities. Mounting on these particular concern is the issue of pandemic-related Medicaid continuous coverage coming to an end. Dr. Mermin of the Center for Disease Control and Prevention’s (CDC) National Center for HIV, Viral Hepatitis, STD and TB Prevention cited “age-old” challenges of “poverty, differences in geographical access, racism, discrimination, homophobia, and stigma,” as layered social and systemic issues that have persisted in furthering the domestic epidemic. Ultimately, the debt ceiling limit agreement sought a clawback of unspent COVID-19 related funding from the CDC which were meant to fill necessary disease intervention specialists and fortify the public health field. As a result, those critically necessary, “boots on the ground” positions may go unfilled.
New Clues as to Why Some People Suppress HIV without Drugs - New research indicates one possibility for why some people’s bodies naturally suppress HIV reproduction. One possible explanation seems to be “abnormally” powerful versions of the infection-fighting white blood cell call CD8+ T cells. These T cells typically live in the lymph nodes. For “spontaneous controllers”, CD8+ T cells appear significantly more adept at identifying and stopping HIV. The estimated incidence of these controllers is about one in every three hundred. Researchers from the Ragon Institute of MGH, MIT and Harvard analyzed blood samples and T cells from seven sero negative patients, nineteen spontaneous controllers and 17 people living with HIV whose viral load was well controlled with antiretroviral therapy. The analysis identified the CD8+ T cells of spontaneous controllers as abundant and highly functional and for the patients on ART, “they are less numerous and less functional”. Reproducing this effect may prove to be challenging with the incidence of qualifying patients being so rare.
Pharmacists Help Build a Model HCV Clinic - During an annual meeting, presenters offered insights from a Washington-based pharmacist-led clinic as a model for how community pharmacies can address Hepatitis C (HCV). The model presents a novel approach in integrating the dispensing pharmacist as a part of HCV care delivery to people who inject drugs. Presenters cautioned careful attention must be given to financial barriers for these types of programs. The model, enacted in 2020, placed pharmacists in a community setting - a syringe exchange program - along with infectious disease providers, substance use treatment outreach workers, and health care navigators once a week. Pharmacists, the presenters argued, could order necessary labs, do assessments and referrals, and dispense curative treatments (as well as help manage other chronic health issues like medication assisted treatment for substance use and HIV management).
Damage to Pfizer Plant Highlights Vulnerable Supply Chain - On July 19th, a tornado touched down in Rocky Mount, North Carolina, destroying a Pfizer plant which produces about 8% of sterile injectables in the United States. The anticipated disruption as a result of the tornado is particularly concerning given the fragility of the United States drug supply chain, as drug shortages are already an issue of concern. The damaged facility, even if not a total loss, could cause months of problems even if other manufacturers were interested and able to take up the capacity needs - for one, the U.S. Food and Drug Administration would have to approve each site for the production of these medications. The issue highlights concerns over the security of the domestic drug supply chain in the face of ever increasing natural disasters and extraordinary heat affecting necessary delivery conditions for many medications.
Assessment of US Pharmacies Contracted with Health Care Institutions under the 340B Drug Pricing Program by Neighborhood Socioeconomic Characteristics - In June 2020, JAMA published an analysis evaluating the physical location of contract pharmacies serving the 340B program and their relative geography to low income patients. The 340B program is a critical funding structure aimed at stretching federal resources to meet the healthcare needs of low-income patients. Among these patients, the impacts of HIV, Hepatitis C (HCV), and substance use disorder (SUD) are higher than wealthier patients and the needs for such funding support more immediate. The analysis found that from 2011 to 2019, the number of 340B retail pharmacies in socioeconomically disadvantaged and primarily non-Hispanic Black and Hispanic/Latino neighborhoods declined. Specifically, the share of 340B pharmacies in disadvantaged neighborhoods decreased by about 5% and the percentage of non-340B pharmacies rose by about 1.3%. In contrast, the share of 340B pharmacies in the highest income neighborhoods increased y about 5% and the percentage of non-340B pharmacies in the highest income neighborhoods increased by about 1.5% There were similar differential declines in neighborhoods with the highest levels of social deprivation.
Congress can Eradicate Hepatitis C and Reduce the Deficit at the Same Time - Neeraj Sood, a senior fellow at the USC Schaeffer Center for Health Policy & Economics and a professor at the USC Price School of Public Policy, along with Jagreet Chhatwal, the director of the Institute for Technology Assessment at Massachusetts General Hospital and associate professor at Harvard Medical School pin an op-ed for STAT News highlighting the economic benefit of making sufficient investments to ending the HCV epidemic in the United States. Leaning into President Biden’s effort to establish a national program to end the HCV epidemic in the US, authors suggest a direct appeal of the public health benefits of ending HCV in the US; preventing 20,000 cases of cancer, 49,100 cases of diabetes, and 25,000 cases of chronic kidney disease over the course of 10 years. Particularly, saving 24,000 lives. They estimate at the ten year mark, that program expenditures for treating tertiary conditions to HCV will result in saving about $18.1 billion. And more than triple those savings at the 20 year mark. With a program cost of $12.3 billion, the argument made focuses on the economic argument for doing the right public health thing.
Using Social Media to Increase HIV Vaccine Trial Participation Among Black Folks - The Body highlights some…modernization of outreach efforts to generate a more equitable participant base for HIV vaccine trials. In partnership with Treatment Action Group, the Black AIDS Institute and the Southern AIDS Coalition with funding provided by the HIV Vaccine Trial Network will be exploring if a stipend and targeted outreach to influencers by way of an ambassador program, might help generate interest in participating in HIV vaccine trials and overcoming medical mistrust in Black communities. But the effort won’t be limited to social media. Organizers are already planning on working to coordinate with high schools and other community spaces in order to extend reach and education.