Watch 03: July 2025
The HIV/HCV Co-Infection Watch is a project of the Community Access National Network (CANN) designed to research, monitor, and report on HIV and Hepatitis C (HCV) co-infection in the United States. The July 2025 Watch includes timely updates herein. To read the project disclaimer and/or methodology, CLICK HERE.
1. FINDINGS
The following is a summary of the key findings for July 2025:
AIDS Drug Assistance Programs:
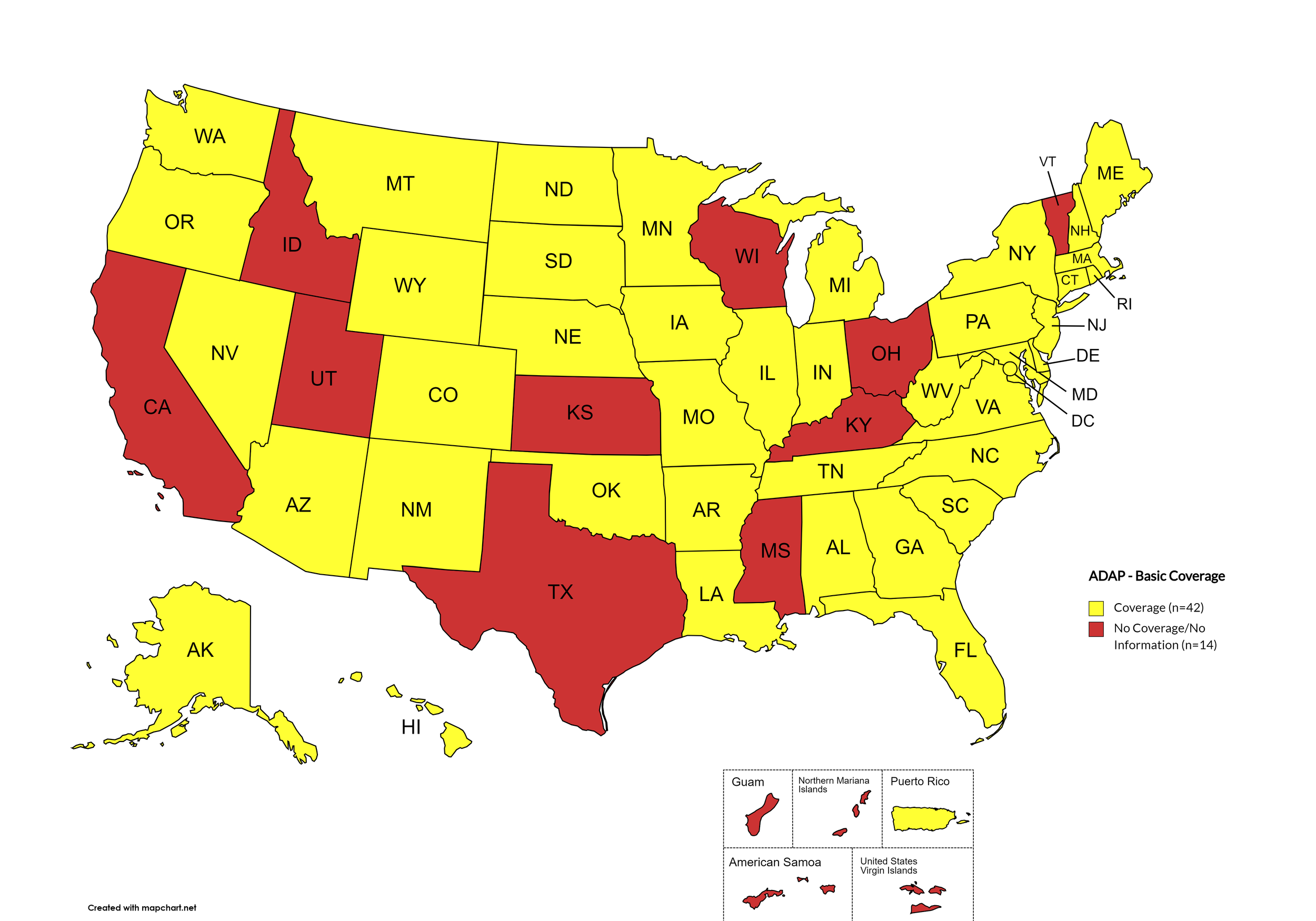
There are 56 state and territorial AIDS Drug Assistance Programs (ADAPs) in the United States, 48 of which offer some form of coverage for Hepatitis C (HCV) treatment. Of those programs, 45 have expanded their HCV coverage to include the Direct-Acting Antiviral (DAA) regimens that serve as the current Standard of Care (SOC) for Hepatitis C treatment. Two (2) programs offer only Basic Coverage, and eight (8) programs offer No Coverage. Three (3) programs cover only select Direct-Acting Antivirals with significant restrictions. Three (3) territories – American Samoa, Marshall Islands, and Northern Mariana Islands – are not accounted for in this data. The most significant federal development was the introduction of the Cure Hepatitis C Act of 2025 in June, proposing a subscription model for HCV medications that could transform access nationwide. A state-by-state Drug Formulary breakdown of coverage is included in the July 2025 Updates, with accompanying drug-specific maps in Figures 1 – 10.
Medicaid Programs:
There are 59 state and territorial Medicaid programs in the United States, and data is represented for all 50 states and the District of Columbia. As of July 2025, all 50 states and the District of Columbia continue to offer Expanded Coverage for hepatitis C treatment. The coverage landscape has seen significant changes since the previous report: Louisiana removed prior authorization requirements for generic HCV medications; Ohio Medicaid began offering HCV treatment for the first time with ribavirin, Mavyret, Pegasys, and generic Epclusa; Nevada substantially reduced its formulary by removing Sovaldi, Harvoni, Zepatier, Vosevi, and generic Harvoni; and South Carolina added Epclusa to its preferred list. Importantly, all state Medicaid programs have maintained their removal of fibrosis restrictions for initial treatment, and no states currently require sobriety as a prerequisite for hepatitis C treatment. Rhode Island remains the only state that does not cover generic Epclusa. A complete state-by-state PDL breakdown of coverage is included in the July 2025 Updates, with accompanying drug-specific maps in Figures 11 – 20.
Patient Assistance Programs:
Our July 2025 analysis of Patient Assistance Programs (PAPs) for hepatitis C treatments reveals mixed developments in program availability. The HealthWell Foundation continues to maintain its reopened Hepatitis C fund with a maximum award of $30,000, providing crucial financial support after its previous closure. However, significant changes occurred with Gilead's Support Path program, which implemented major modifications on May 5, 2025, transitioning from retail pharmacy to mail order delivery and discontinuing free medications for several products due to generic availability. The Patient Advocate Foundation's Co-Pay Relief program closed to new HCV applications, while The Assistance Fund moved to waitlist status. The PAN Foundation adjusted grant amounts beginning January 1, 2025, in response to Medicare Part D's new $2,000 out-of-pocket cap. These changes reflect the evolving landscape of patient assistance as generic medications become more widely available and federal healthcare policies shift.
Harm Reduction Programs:
Syringe Services Programs: Forty-three (43) states, the District of Columbia and two (2) territories currently have Syringe Services Programs (SSPs) in place, regardless of the legality. Seven (7) states without SSPs are Alabama, Idaho, Kansas, Mississippi, Nebraska, South Dakota, and Wyoming. Idaho's situation reflects the most significant change, having repealed its Syringe and Needle Act (House Bill 617) effective July 1, 2024, becoming the first state to completely eliminate authorized syringe services programs.
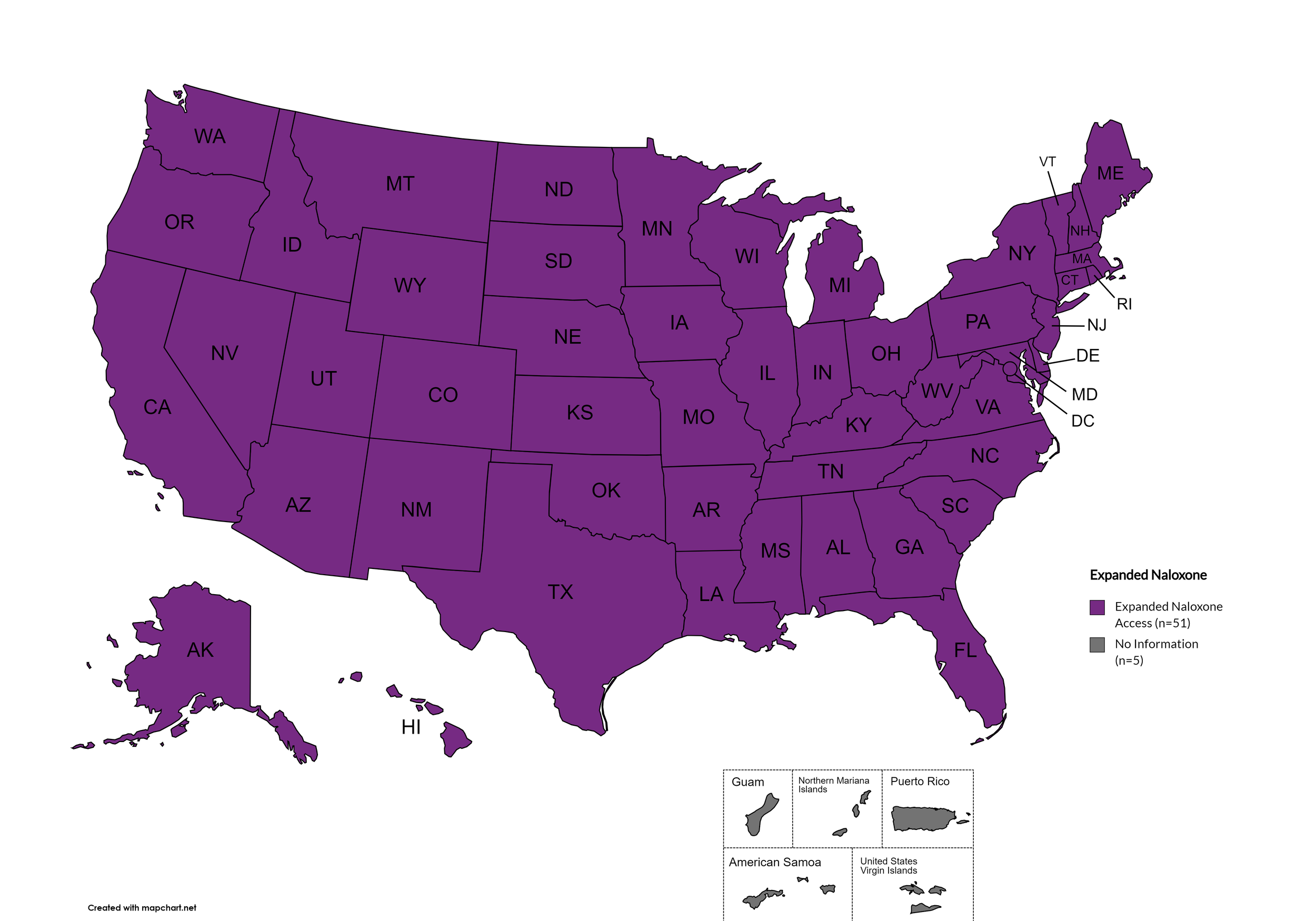
Expanded Naloxone Access: All fifty (50) states and the District of Columbia have health department distribution programs for Naloxone and/or allow Medicaid coverage of Naloxone. No states have restricted naloxone access, with innovative distribution methods emerging including vending machine programs in Denver (2,100+ boxes distributed), St. Louis County (44 new machines), and California's direct-to-consumer CalRx program offering naloxone at $24 per twin-pack.
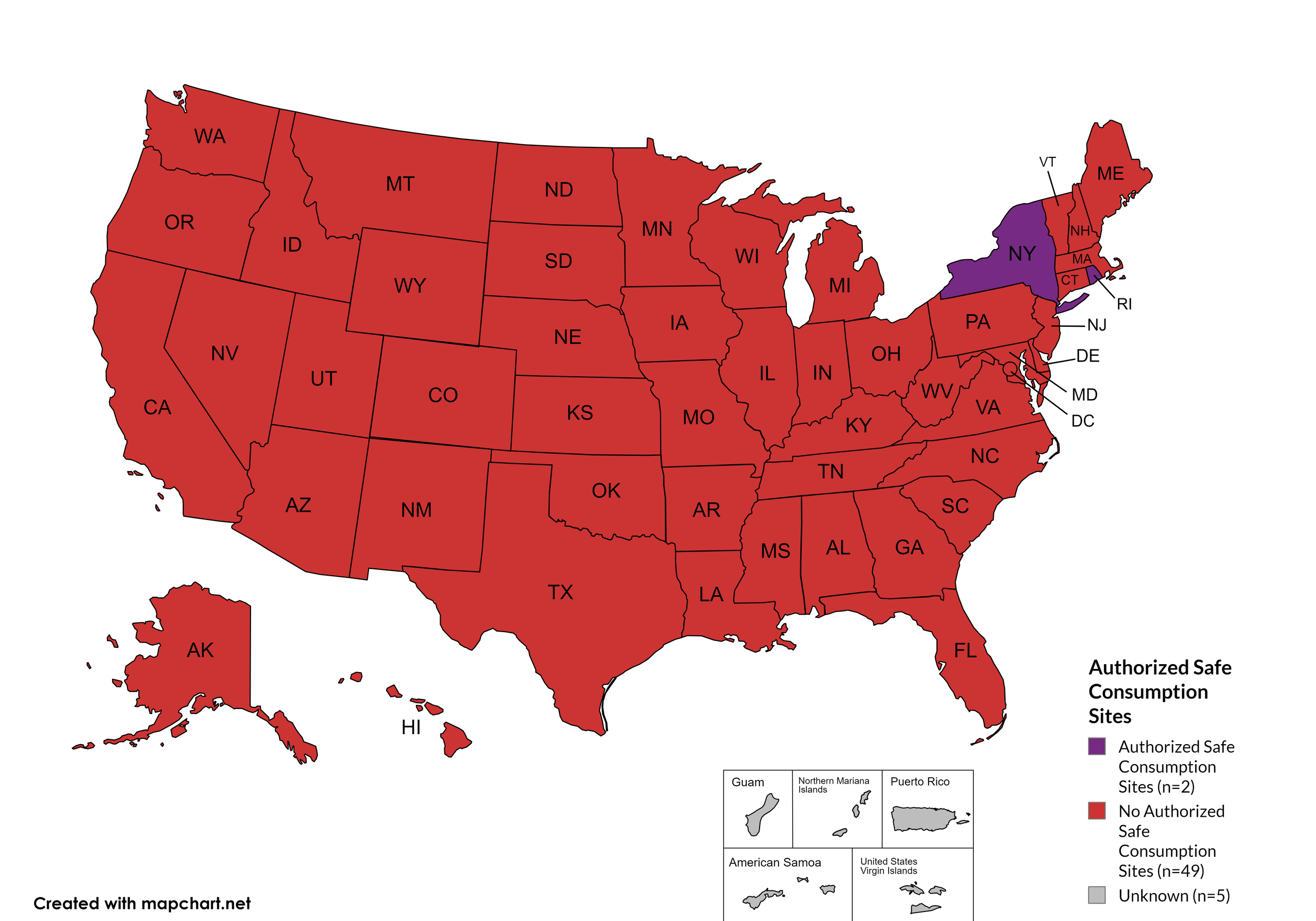
Safe Consumption Sites: Two (2) states explicitly authorize or have authorized pilot projects for Safe Consumption Sites (SCSs): New York (operational since November 2021) and Rhode Island (opened December 2024). Rhode Island's first state-sanctioned overdose prevention center reported over 500 visitors and 27 overdose deaths prevented in its first six months of operation. The legislature approved a two-year extension of the pilot program in April 2025.
Updated Paraphernalia Laws: Forty-nine (49) states have modernized their criminal codes to allow for possession of testing strips and may also have health department programs distributing testing strips. Indiana decriminalized fentanyl test strips effective July 1, 2025, through House Bill 1167. Iowa remains the only state where testing strips are still explicitly categorized as illegal paraphernalia.
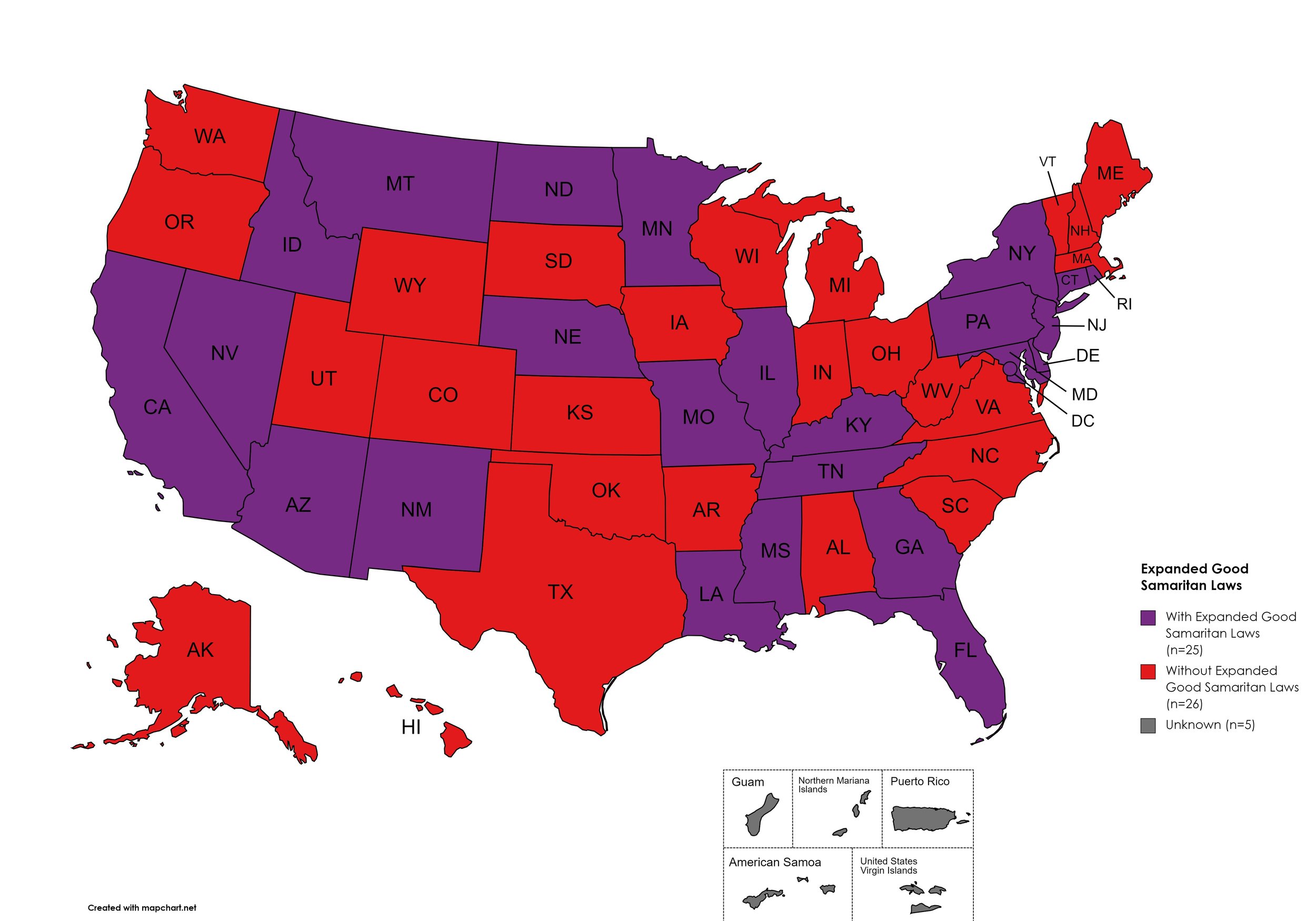
Good Samaritan Laws: Twenty-five (25) states have Good Samaritan laws or statutes that provide some level of protection for those seeking or giving assistance during a drug overdose, regardless of possession of controlled substance or consumption of illegal or illicit substances. No new states added Good Samaritan protections during the research period.
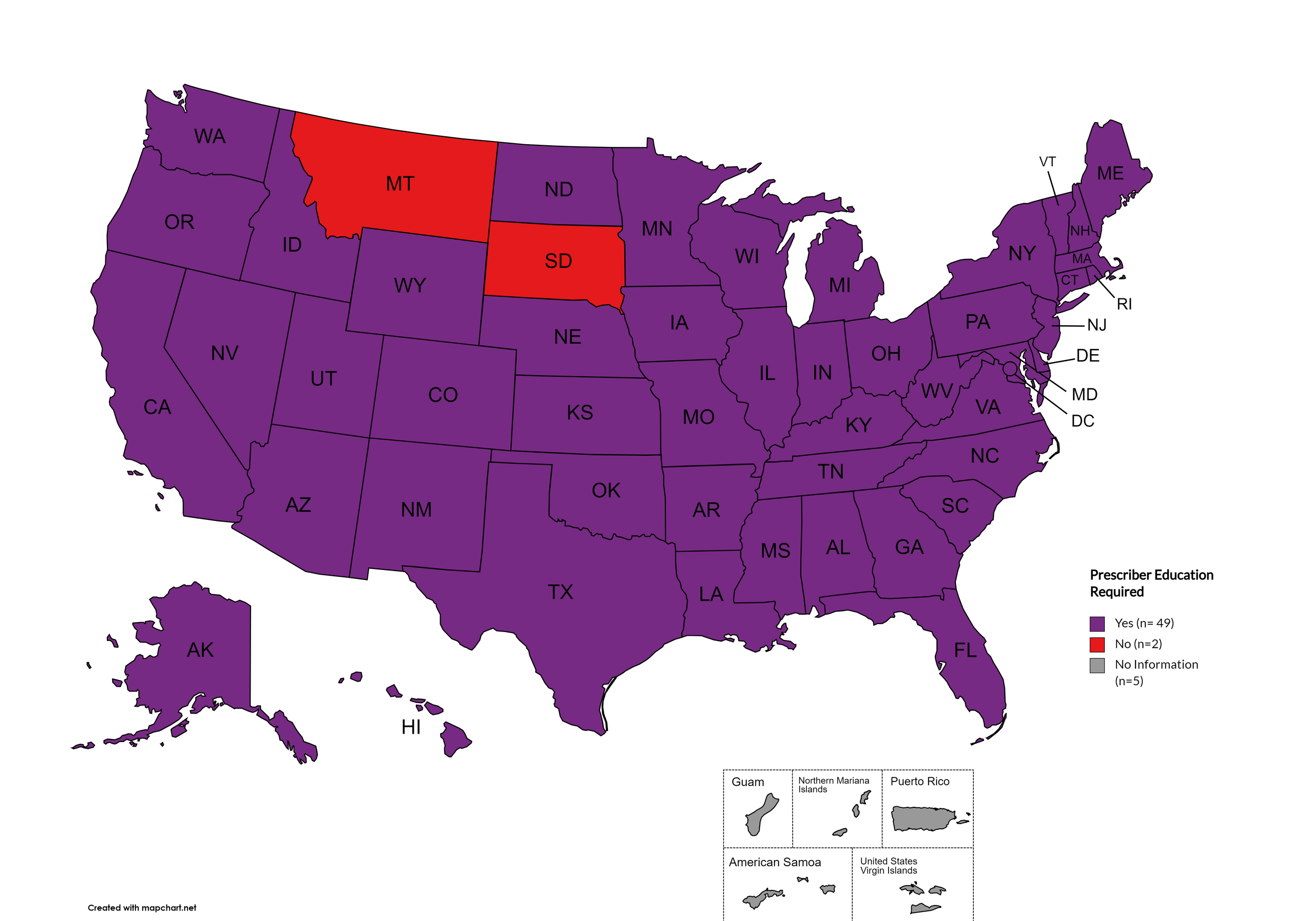
Prescriber Education: Forty-eight (48) states and the District of Columbia require, through legislative action or regulatory or licensing bodies, that prescribing physicians attend mandatory and continuing opioid prescribing or harm reduction education sessions. Montana and South Dakota remain the only states without these requirements. A state-by-state program breakdown is included in the July 2025 Updates, with accompanying maps in Figures 21-26.
2. AIDS DRUG ASSISTANCE PROGRAMS (ADAPs) & HCV THERAPIES
Of the 56 respective state and territorial ADAPs, only 8 (KS, KY, OH, UT, VT, GU, PW, VI) do not offer any coverage for HCV drug therapies. States whose formularies are not available on the state-run website have been checked against the most recent National Alliance of State and Territorial AIDS Directors (NASTAD) formulary database (last updated January 1, 2025). The data presented are current as of July 25, 2025.
July 2025 Updates:
Basic Coverage
States with Basic HCV Medications Coverage: AL, AK, AZ, AR, CO, CT, DE, FL, GA, HI, IL, IN, IA, LA, ME, MD, MA, MI, MN, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OR, PA, RI, SC, SD, TN, VA, WA, WV, WY, D.C.
States without Basic HCV Medications Coverage: CA, ID, KS, KY, MS, OH, TX, UT, VT, WI
Territories with Basic HCV Medications Coverage: P.R.
Figure 1. July 2025 ADAP Coverage - Basic
Map Key: Yellow = Basic Coverage; Red = No Basic Coverage/No Information regarding Basic Coverage
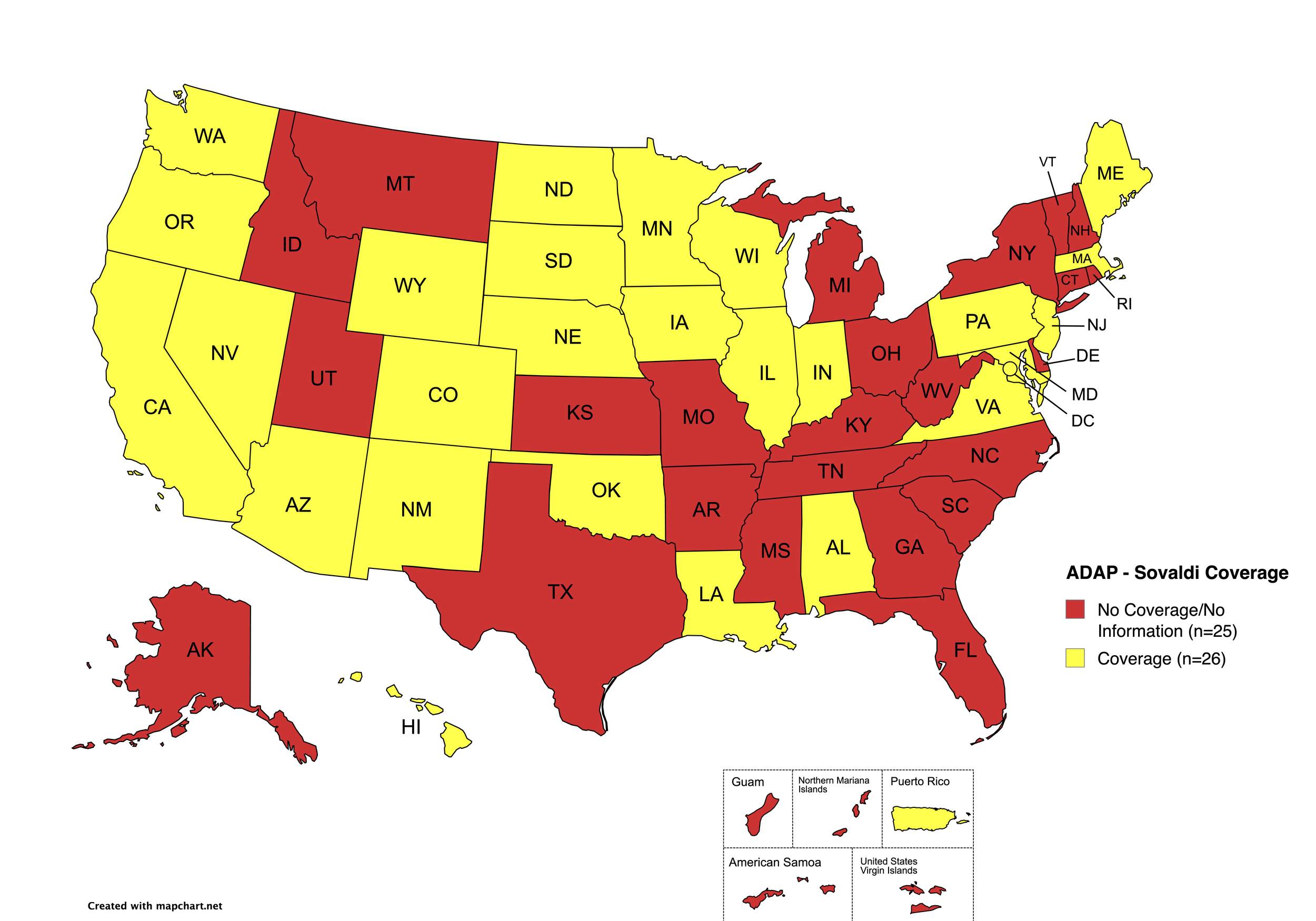
Sovaldi
States with Sovaldi Coverage: AZ, CA, CO, HI, IL, IN, IA, LA, ME, MD, MA, MN, NE, NV, NJ, NM, ND, OK, OR, PA, SD, VA, WA, WI, WY, D.C.
States without Sovaldi Coverage: AL, AK, AR, CT, DE, FL, GA, ID, KS, KY, MI, MS, MO, MT, NH, NY, NC, OH, RI, SC, TN, TX, UT, VT, WV
Territories with Sovaldi Coverage: P.R.
Figure 2. July 2025 ADAP Coverage - Sovaldi
Map Key: Yellow = Sovaldi Coverage; Red = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
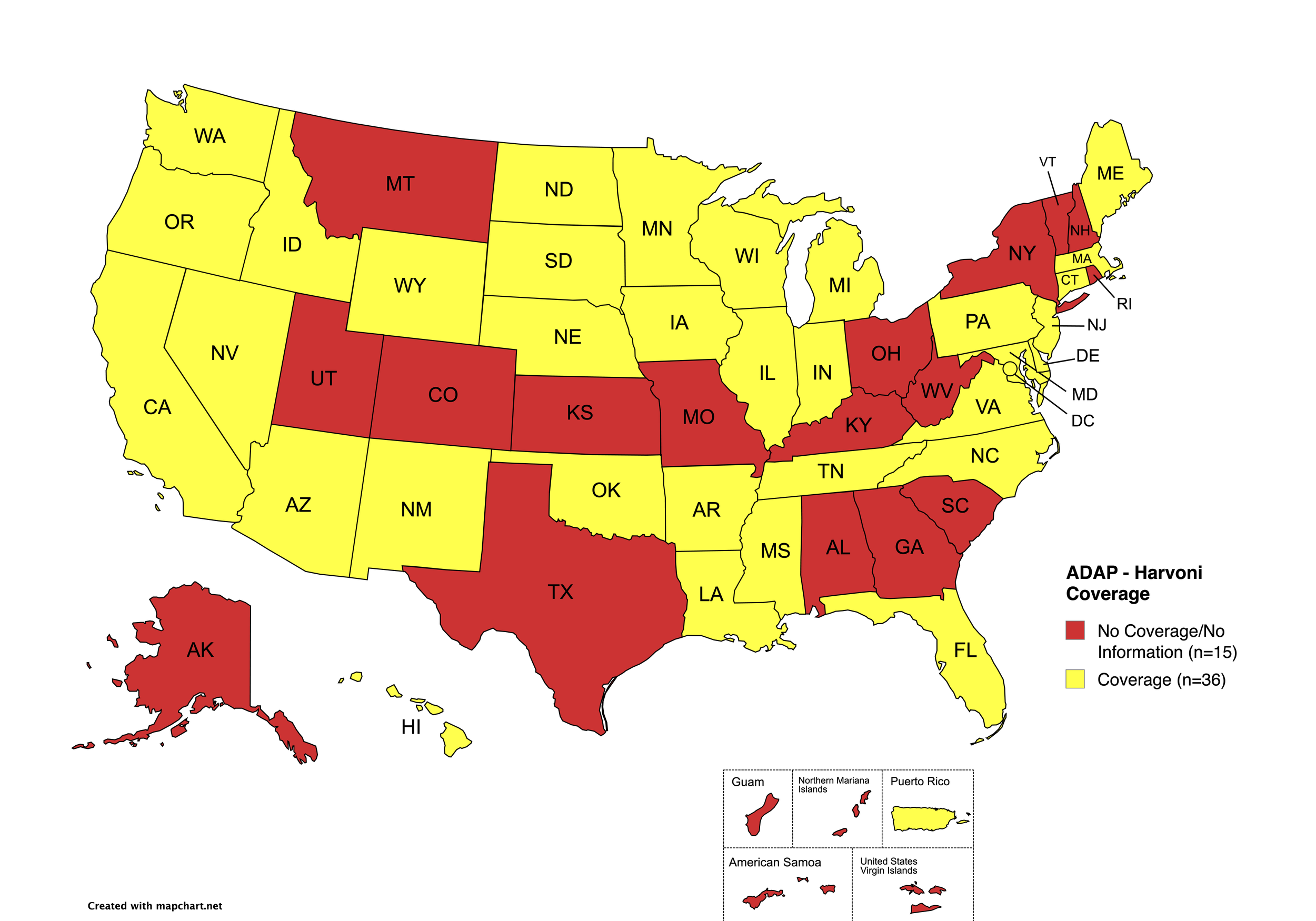
Harvoni
States with Harvoni Coverage: AZ, AR, CA, CO, CT, DE, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, NE, NV, NJ, NM, NC, ND, OK, OR, PA, SD, TN, VA, WA, WI, WY, D.C.
States without Harvoni Coverage: AL, AK, GA, KS, KY, MO, MT, NH, NY, OH, RI, SC, TX, UT, VT, WV
Territories with Harvoni Coverage: P.R.
Figure 3. July 2025 ADAP Coverage - Harvoni
Map Key: Yellow = Harvoni Coverage; Red = No Harvoni Coverage/No Information regarding Harvoni Coverage
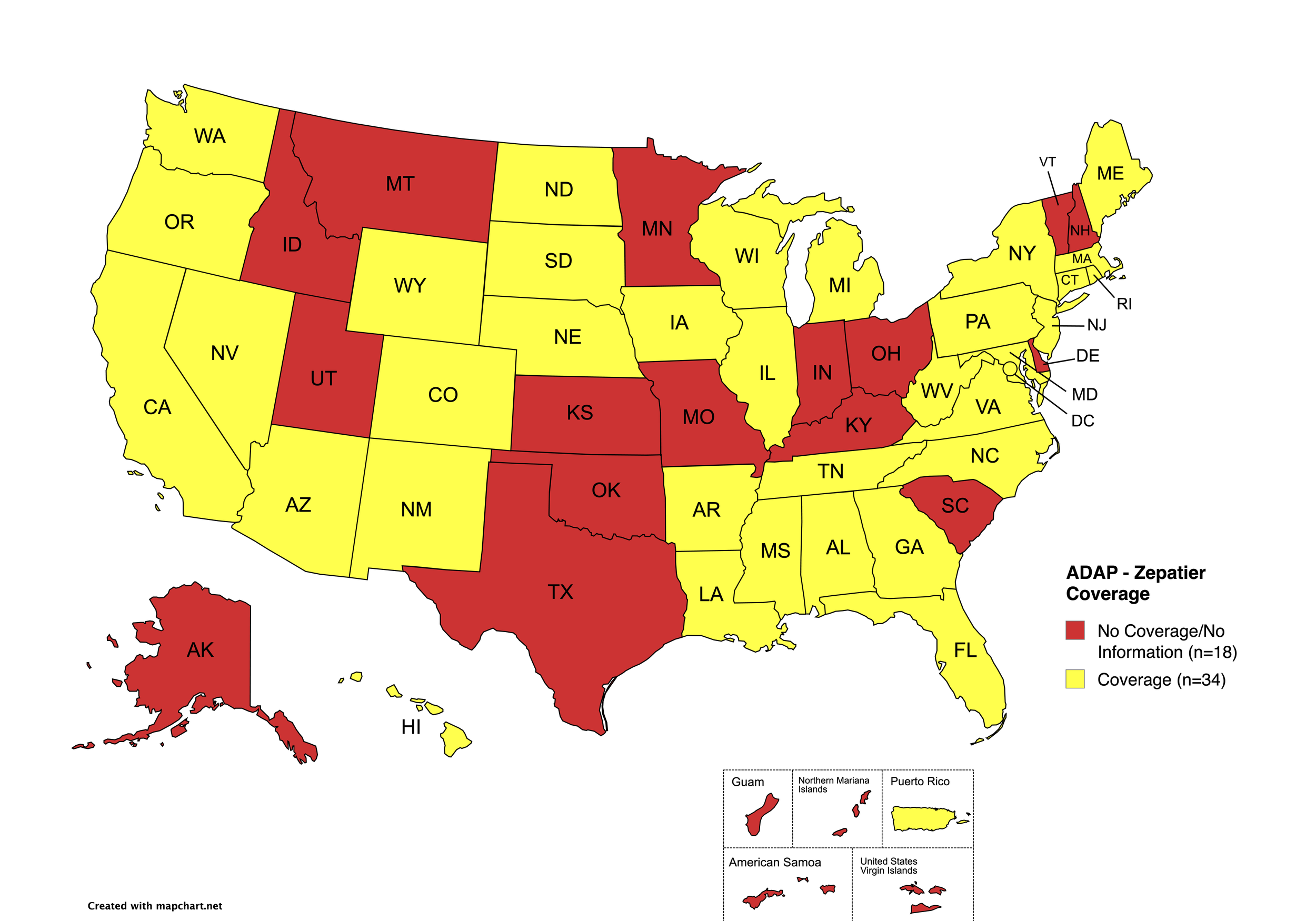
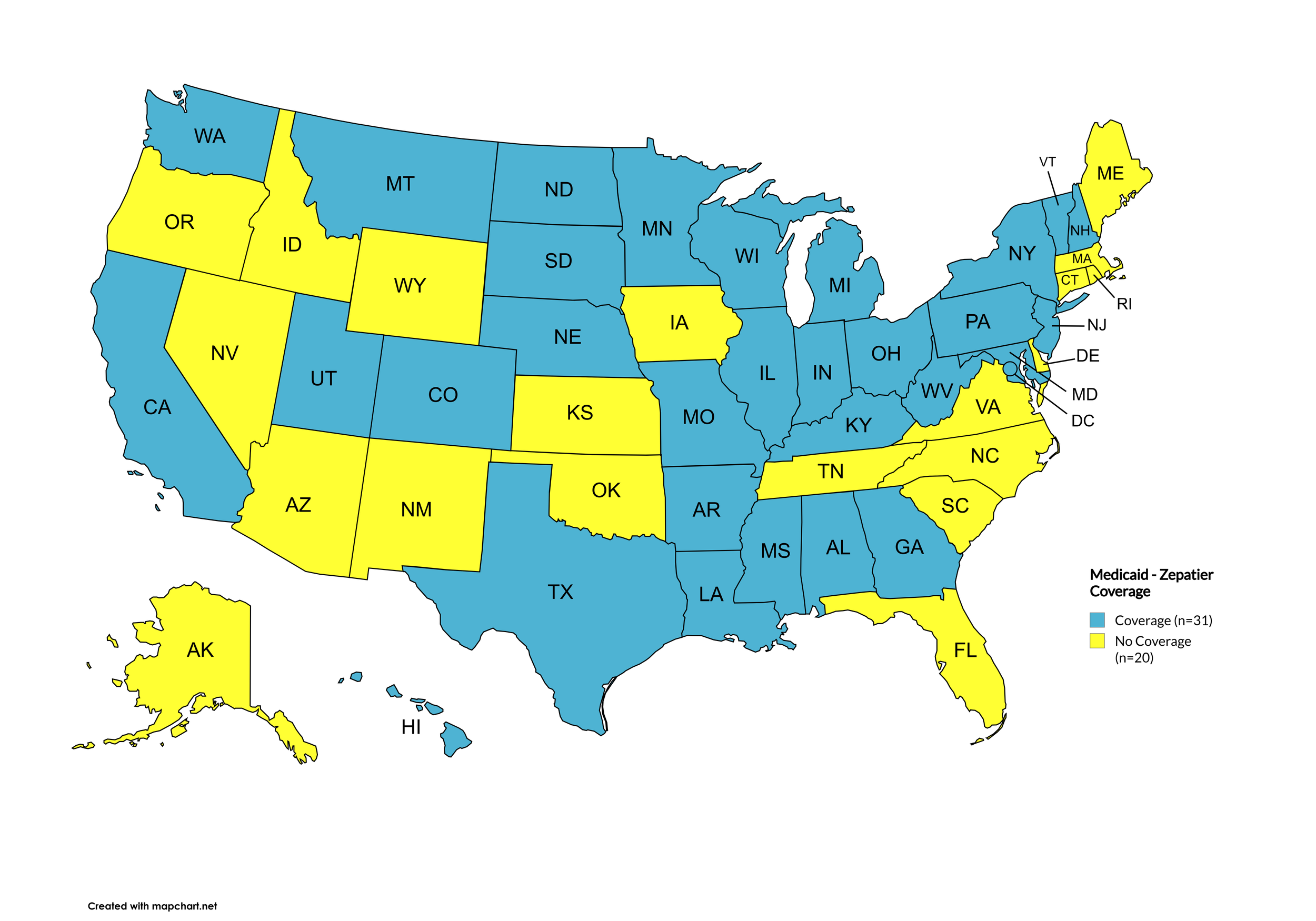
Zepatier
States with Zepatier Coverage: AL, AZ, AR, CA, CO, FL, GA, HI, IL, IA, LA, ME, MD, MA, MI, MS, NE, NV, NJ, NM, NY, NC, ND, OR, PA, SD, VA, WA, WV, WI, WY, D.C.
States without Zepatier Coverage: AK, CT, DE, ID, IN, KS, KY, MN, MO, MT, NH, OH, OK, RI, SC, TN, TX, UT, VT
Territories with Zepatier Coverage: P.R.
Figure 4. July 2025 ADAP Coverage - Zepatier
Map Key: Yellow = Zepatier Coverage; Red = No Zepatier Coverage/No Information regarding Zepatier Coverage
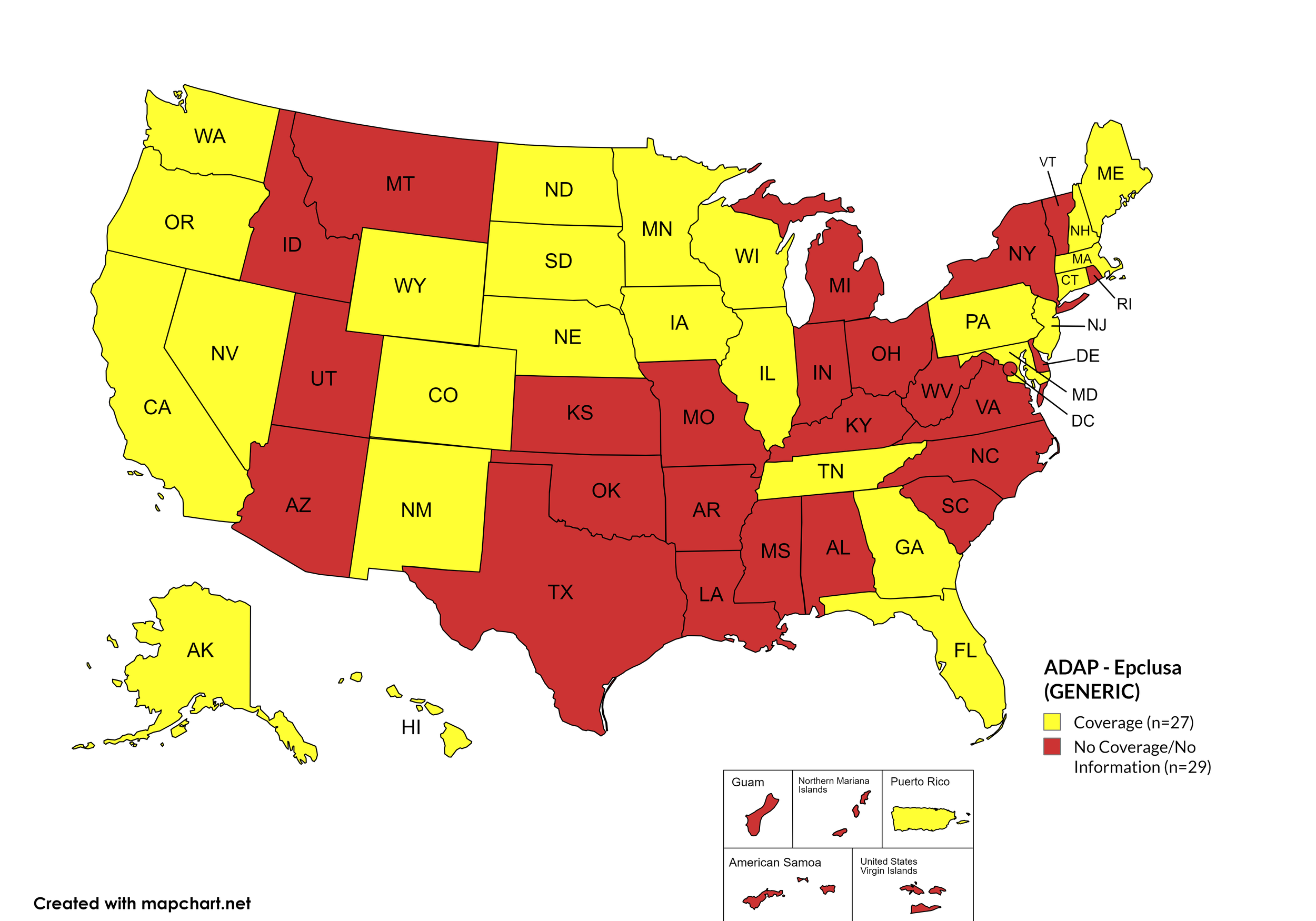
Epclusa
States with Epclusa Coverage: AZ, AR, CA, CO, CT, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, NE, NV, NJ, NM, ND, OR, PA, SD, TN, VA, WA, WI, WY
States without Epclusa Coverage: AL, AK, DE, GA, KS, KY, MT, NH, NY, NC, OH, OK, RI, SC, TX, UT, VT, WV, D.C.
Territories with Epclusa Coverage: P.R.
Figure 5. July 2025 ADAP Coverage - Epclusa
Map Key: Yellow = Epclusa Coverage; Red = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: CA, CO, CT, FL, HI, ID, IL, IN, IA, LA, ME, MD, MA, NE, NV, NJ, NM, ND, OR, SD, TN, WA, WY
States without Vosevi Coverage: AL, AK, AZ, AR, DE, GA, KS, KY, MI, MN, MS, MO, MT, NH, NY, NC, OH, OK, PA, RI, SC, TX, UT, VT, VA, WV, WI, D.C.
Territories with Vosevi Coverage: P.R.
Figure 6. July 2025 ADAP Coverage - Vosevi
Map Key: Yellow = Vosevi Coverage; Red = No Vosevi Coverage/No Information regarding Vosevi Coverage
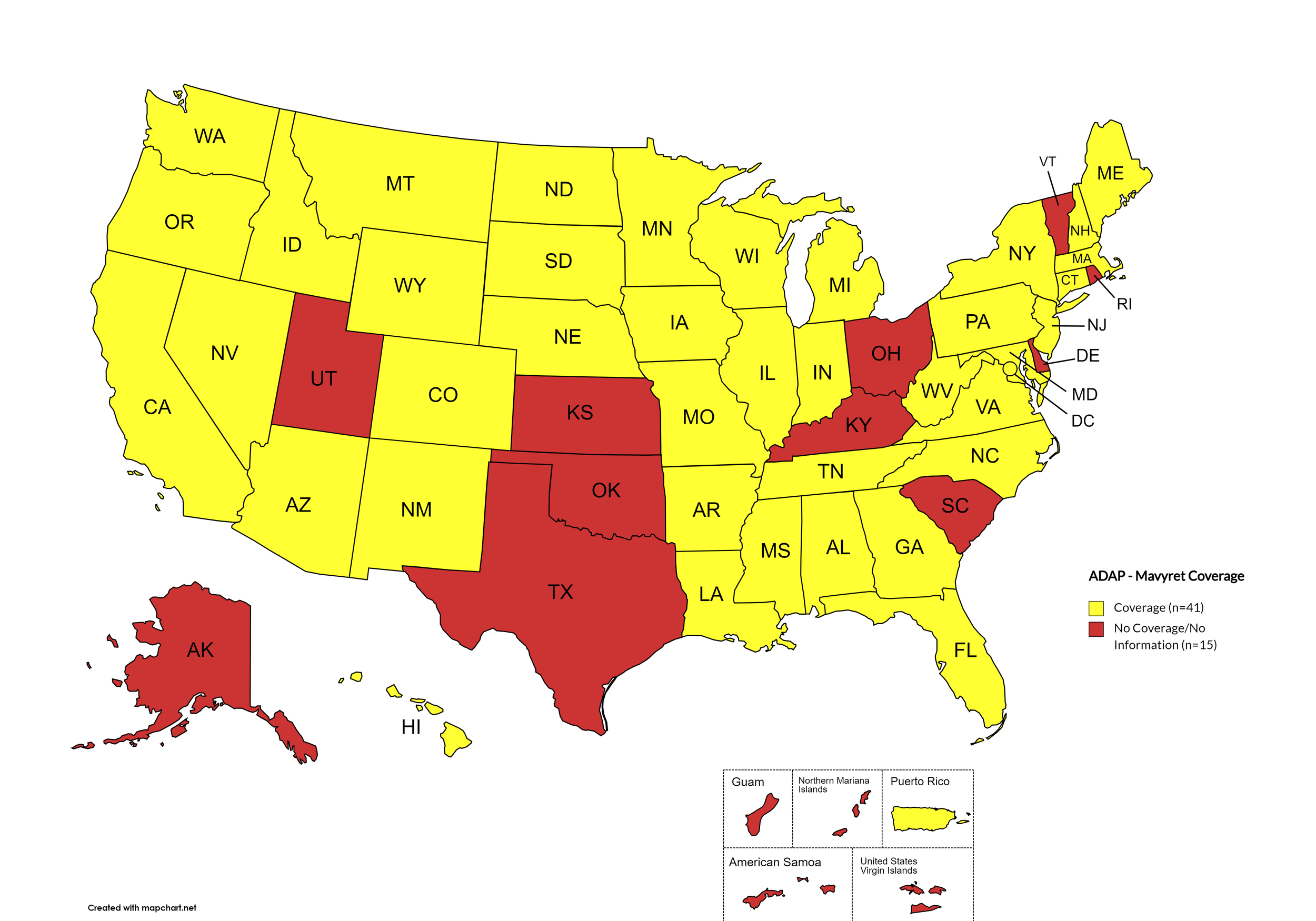
Mavyret
States with Mavyret Coverage: AL, AZ, AR, CA, CO, CT, FL, GA, HI, ID, IL, IN, IA, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NJ, NM, NY, NC, ND, OR, PA, SD, TN, VA, WA, WV, WI, WY, D.C.
States without Mavyret Coverage: AK, DE, KS, KY, NH, OH, OK, RI, SC, TX, UT, VT
Territories with Mavyret Coverage: P.R.
Figure 7. July 2025 ADAP Coverage - Mavyret
Map Key: Yellow = Mavyret Coverage; Red = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AL, CA, CT, DE, HI, IA, LA, ME, MD, MA, MI, MN, NE, NV, NJ, NM, NC, ND, OH, OR, PA, RI, SD, WA, WV, WI, WY, D.C.
States without Pegasys Coverage: AK, AZ, AR, CO, FL, GA, ID, IN, KS, KY, MS, MO, MT, NH, NY, OH, OK, SC, TN, TX, UT, VT, VA
Territories with Pegasys Coverage: None/Unknown
Figure 8. July 2025 ADAP Coverage - Pegasys
Map Key: Yellow = Pegasys Coverage; Red = No Pegasys Coverage/No Information regarding Pegasys Coverage
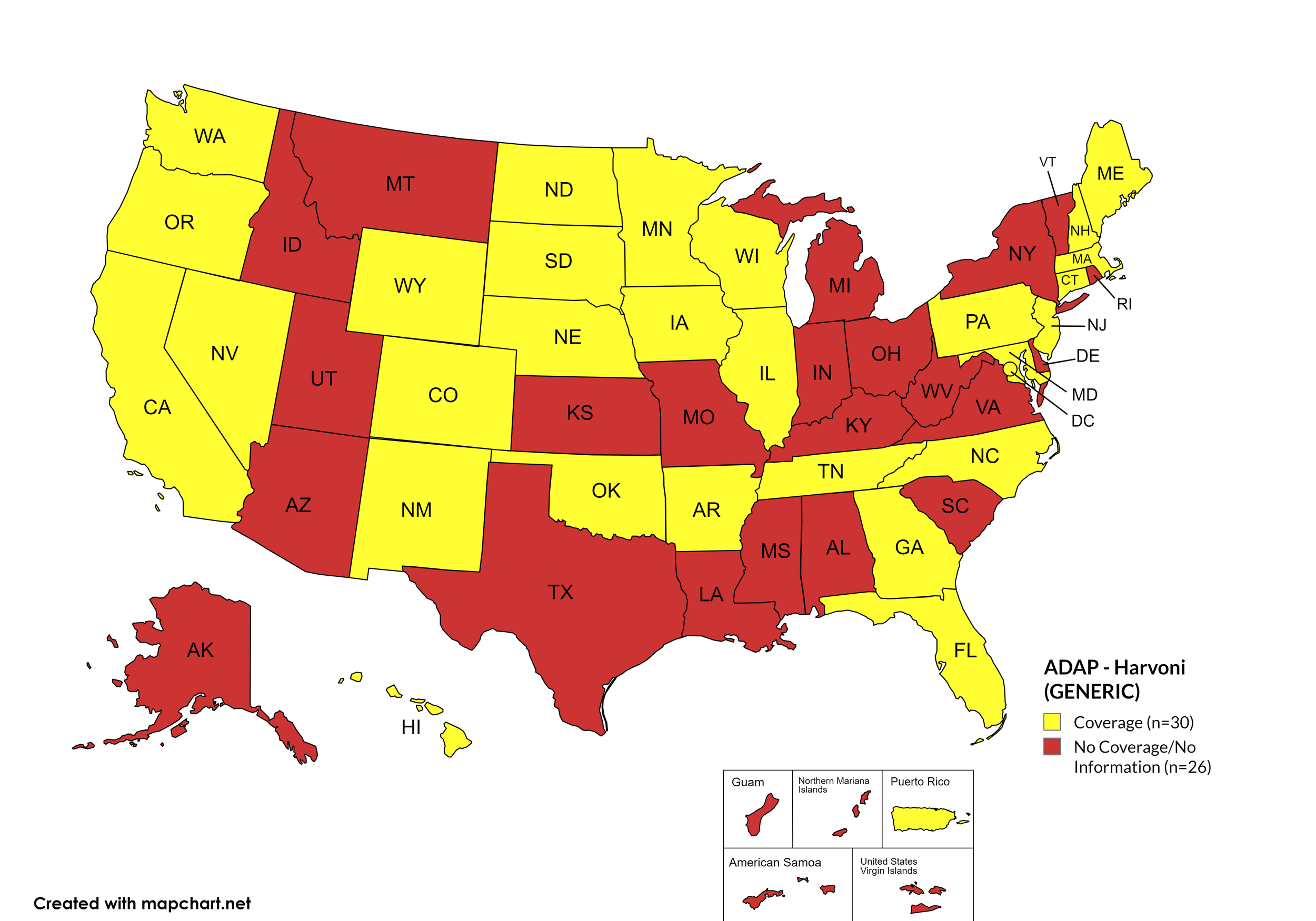
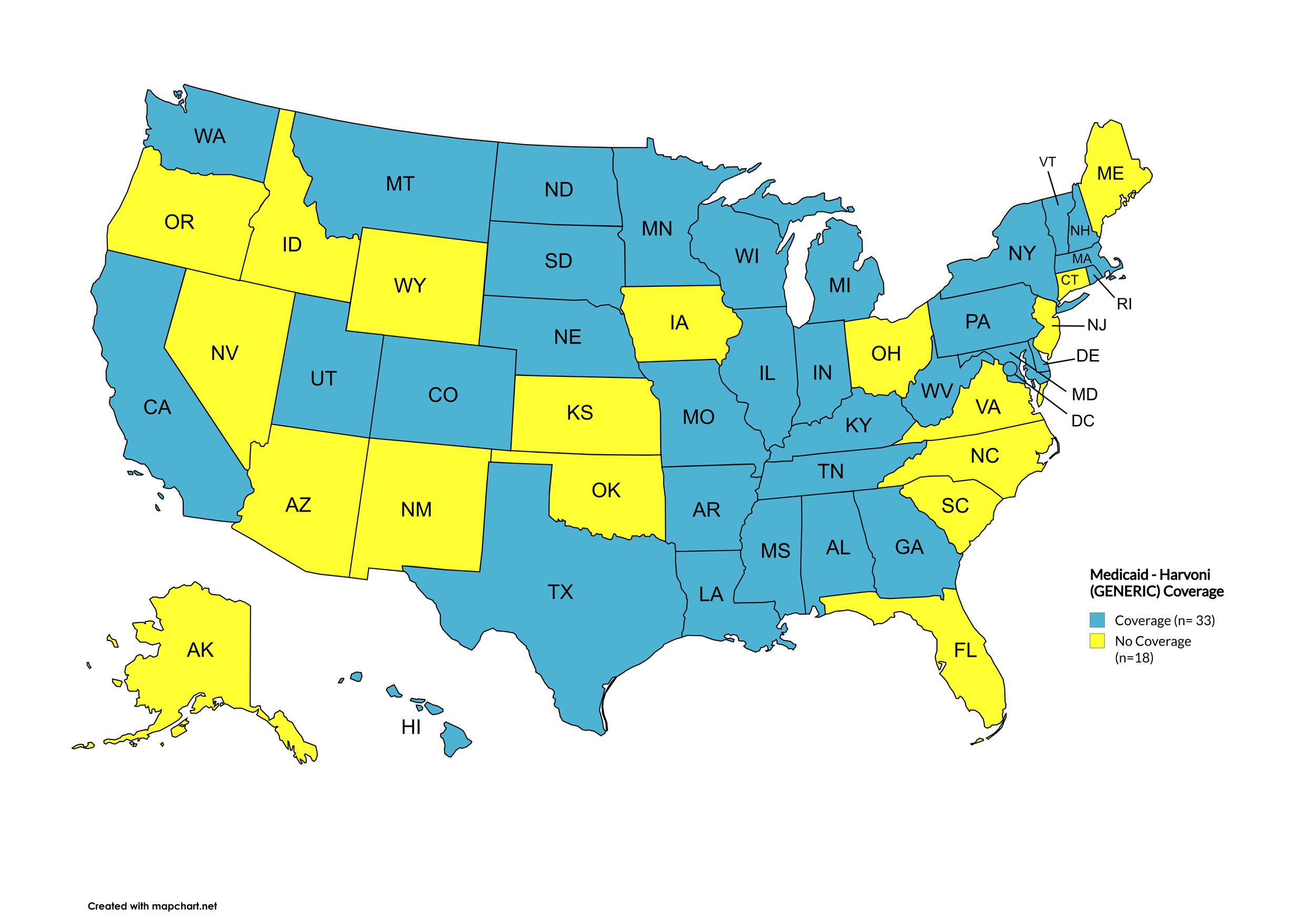
Harvoni (generic)
States with Harvoni (generic) Coverage: AR, CA, CO, CT, FL, GA, HI, IL, IA, ME, MD, MA, MN, NE, NV, NJ, NM, NC, ND, OK, OR, PA, SD, TN, WA, WI, WY, D.C.
States without Harvoni (generic)Coverage: AL, AK, AZ, DE, ID, IN, KS, KY, LA, MI, MS, MO, MT, NH, NY, OH, RI, SC, TX, UT, VT, VA, WV
Territories with Harvoni (generic) Coverage: P.R.
Figure 9. July 2025 ADAP Coverage - Harvoni (Generic)
Map Key: Yellow = Harvoni (Generic) Coverage; Red = No Harvoni (Generic) Coverage/No Information regarding Harvoni (Generic) Coverage
Epclusa (generic)
States with Epclusa (generic) Coverage: AR, CA, CO, CT, FL, GA, HI, IL, IA, ME, MD, MA, MN, NE, NV, NJ, NM, ND, OR, PA, SD, TN, WA, WI, WY
States without Epclusa (generic) Coverage: AL, AK, AZ, DE, ID, IN, KS, KY, LA, MS, MO, MI, MT, NH, NY, NC, OH, OK, RI, SC, TX, UT, VT, VA, WV, D.C.
Territories with Epclusa (generic) Coverage: P.R.
Figure 10. July 2025 ADAP Coverage - Epclusa (generic)
Map Key: Yellow = Epclusa (generic) Coverage; Red = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
July 2025 Notes:
States with Open Formularies: IL, IA, MA, MN, NE, NH, NJ, NM, ND, OH, OR, WA, WY
N.B. – Although Ohio is listed by NASTAD as having an open formulary, both NASTAD’s ADAP Formulary Database and Ohio’s ADAP website indicates that the state does not offer any treatment for HCV.
N.B. – Although North Dakota has adopted an open formulary, they provide only co-pay and deductible assistance for HCV medications.
N.B. – Wyoming's ADAP Open Formulary document, the following disclaimer related to HCV is made: Hepatitis C treatment medications (i.e. Harvoni, Sovaldi, Ribavirin, Zepatier, Epclusa) must be prior authorized. To be eligible, clients must have applied for prior authorization from their insurance plan and the WY ADAP Hepatitis C Treatment checklist must be completed and signed by the provider and client.
Colorado offers five coverage options – Standard ADAP, HIV Medical Assistance Program (HMAP), Bridging the Gap Colorado (BTGC), HIV Insurance Assistance Program (HIAP), and Supplemental Wrap Around Program (SWAP). 'Yes' indications in Figure 1 for Colorado denote that at least one of these programs offers coverage for each respective drug. Coverage through the Standard ADAP Formulary remains subject to funding availability.
On August 11th, 2023, Georgia's Department of Public Health issued a notice to Ryan White Part B District Coordinators, reading, in part, "Effective 8/14/2023, care providers will have the ability to order Hepatitis C medications for their eligible ADAP patients without the need for Prior Approval." Initially covered medications are limited to ribavirin, Zepatier, Mavyret, and generics for Epclusa and Harvoni.
Hawaii's ADAP notes the following: "Treatment slots for HCV direct-acting antivirals may be limited. Prescriber or pharmacy must call HDAP for slot."
Louisiana's ADAP (Louisiana Health Access Program – LA HAP) offers two coverage options – Uninsured (Louisiana Drug Assistance Program – L-DAP) and Insured (Health Insurance Program – HIP). HIP pays for the cost of treatment only if the client's primary insurance covers the drug under its formulary.
New Hampshire updated its formulary to require step therapy for Sovaldi, Harvoni, Zepatier, Epclusa, and Vosevi effective July 1, 2025. Preferred drugs are ribavirin, Pegasys, Mavyret, generic Harvoni, and generic Epclusa. This change affects coverage for brand-name DAAs, requiring patients to try preferred medications first.
Texas's ADAP maintains no HCV coverage, despite a brief period of covering DAAs in 2022.
Federal Legislative Development: The Cure Hepatitis C Act of 2025 (S.1941), introduced June 4, 2025, by Senators Bill Cassidy (R-LA) and Chris Van Hollen (D-MD), proposes establishing a national HCV elimination program with subscription-based drug access. NASTAD collaborated in drafting this bipartisan legislation, which could significantly expand treatment access if enacted.
NASTAD Database Status: The National Alliance of State and Territorial AIDS Directors formulary database remains current as of January 1, 2025, with no updates issued during the April-July 2025 period. Discrepancies between state websites and the database continue to create monitoring challenges.
3. MEDICAID PROGRAMS & HCV THERAPIES
All 50 states and the District of Columbia continue to offer some form of HCV coverage. All 50 States and the District of Columbia have expanded their Preferred Drug Lists to include at least one HCV Direct Acting Agent (DAA).
July 2025 Updates:
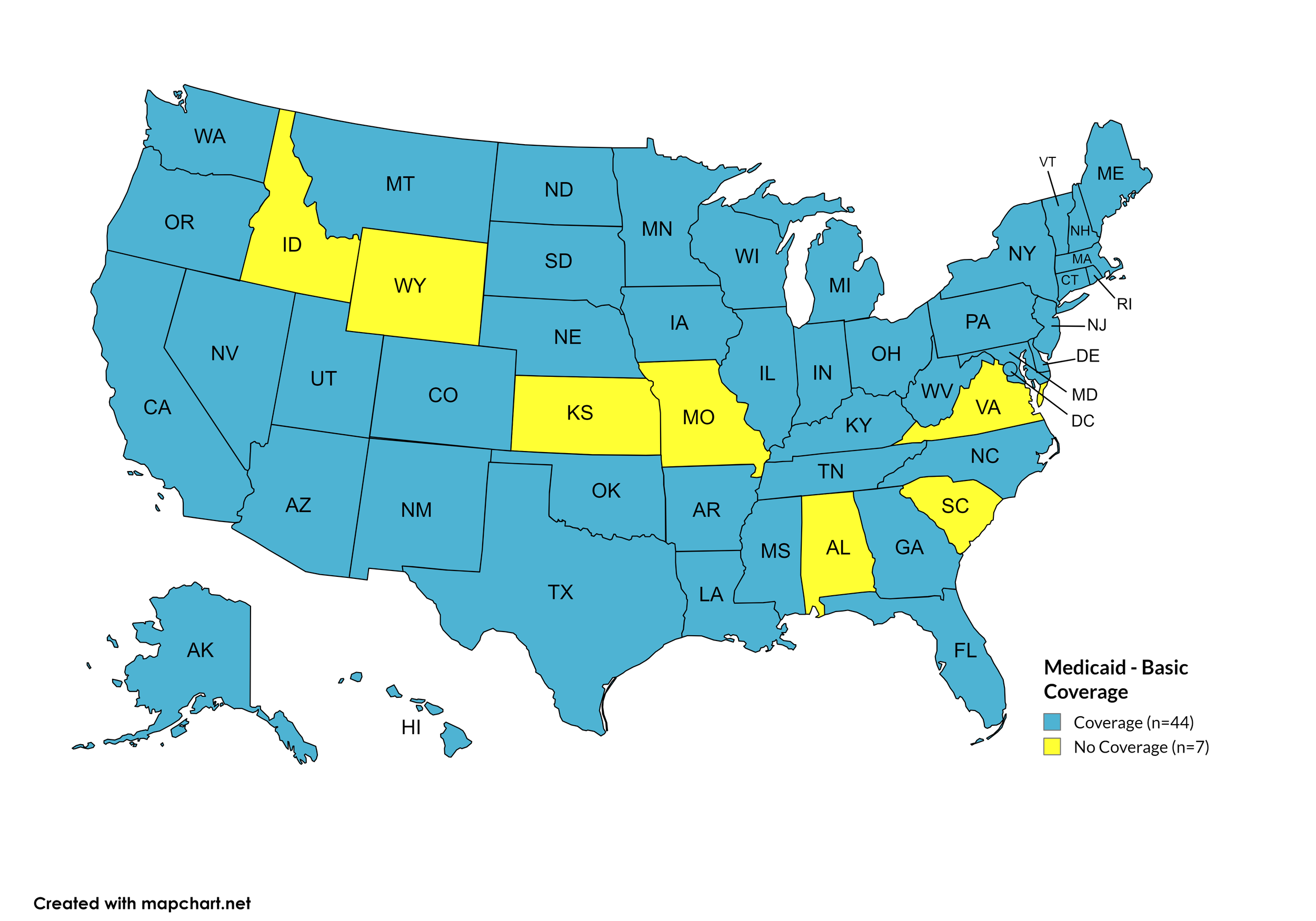
Basic Coverage
States with Basic HCV Medications Coverage: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, WY, D.C.
States without Basic HCV Medications Coverage: AL, ID, KS, MO, SC, VA
Figure 11. July 2025 Medicaid Coverage - Basic HCV Medications
Map Key: Blue = Basic HCV Medication Coverage; Yellow = No Basic HCV Medication Coverage/No Information regarding Basic HCV Medication Coverage
Sovaldi
States with Sovaldi Coverage: AR, CA, CO, GA, HI, IL, IN, KY, LA, MD, MA, MI, MN, MS, MO, MT, NE, NY, ND, PA, RI, SD, TX, UT, VT, WA, WI, D.C.
States without Sovaldi Coverage: AK, AL, AZ, CT, DE, FL, ID, IA, KS, ME, NH, NJ, NM, NV, NC, OK, OH, OR, SC, TN, VA, WV, WY
Figure 12. July 2025 Medicaid Coverage - Sovaldi
Map Key: Blue = Sovaldi Coverage; Yellow = No Sovaldi Coverage/No Information regarding Sovaldi Coverage
Harvoni
States with Harvoni Coverage: AL, AR, CA, CO, GA, HI, IL, IN, KY, LA, MI, MN, MS, MO, MT, NE, NH, NY, ND, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni Coverage: AK, AZ, CT, DE, FL, ID, IA, KS, ME, MD, MA, NV, NJ, NM, NC, OK, OH, OR, SC, VA, WY
Figure 13. July 2025 Medicaid Coverage - Harvoni
Map Key: Blue = Harvoni Coverage; Yellow = No Harvoni Coverage/No Information regarding Harvoni Coverage
Zepatier
States with Zepatier Coverage: AL, AR, CA, CO, GA, HI, IL, IN, KY, LA, MD, MI, MN, MS, MO, MT, NE, NH, NJ, NY, ND, OH, PA, SD, TX, UT, VT, WA, WI, WV, D.C.
States without Zepatier Coverage: AK, AZ, CT, DE, FL, ID, IA, KS, ME, MA, NV, NM, NC, OK, OR, RI, SC, TN, VA, WY.
Figure 14. July 2025 Medicaid Coverage - Zepatier
Map Key: Blue = Zepatier Coverage; Yellow = No Zepatier Coverage/No Information regarding Zepatier Coverage
Epclusa
States with Epclusa Coverage: AL, CA, CO, GA, HI, IL, IN, KY, LA, MD, MI, MN, MO, MS, MT, NH, NJ, NY, ND, PA, SC, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Epclusa Coverage: AK, AZ, AR, CT, DE, FL, ID, IA, KS, ME, MA, NE, NV, NM, NC, OH, OK, OR, RI, VA, WY
Figure 15. July 2025 Medicaid Coverage - Epclusa
Map Key: Blue = Epclusa Coverage; Yellow = No Epclusa Coverage/No Information regarding Epclusa Coverage
Vosevi
States with Vosevi Coverage: AR, CO, CT, FL, GA, HI, ID, IL, IN, KY, LA, MD, MI, MN, MO, MS, MT, NE, NH, NY, NC, ND, OH, PA, SC, SD, TX, UT, VT, WA, WV, WI, D.C.
States without Vosevi Coverage: AK, AL, AZ, CA, DE, IA, KS, NV, NJ, NM, ME, MA, OK, OR, RI, TN, VA, WY
Figure 16. July 2025 Medicaid Coverage - Vosevi
Map Key: Blue = Vosevi Coverage; Yellow = No Vosevi Coverage/No Information regarding Vosevi Coverage
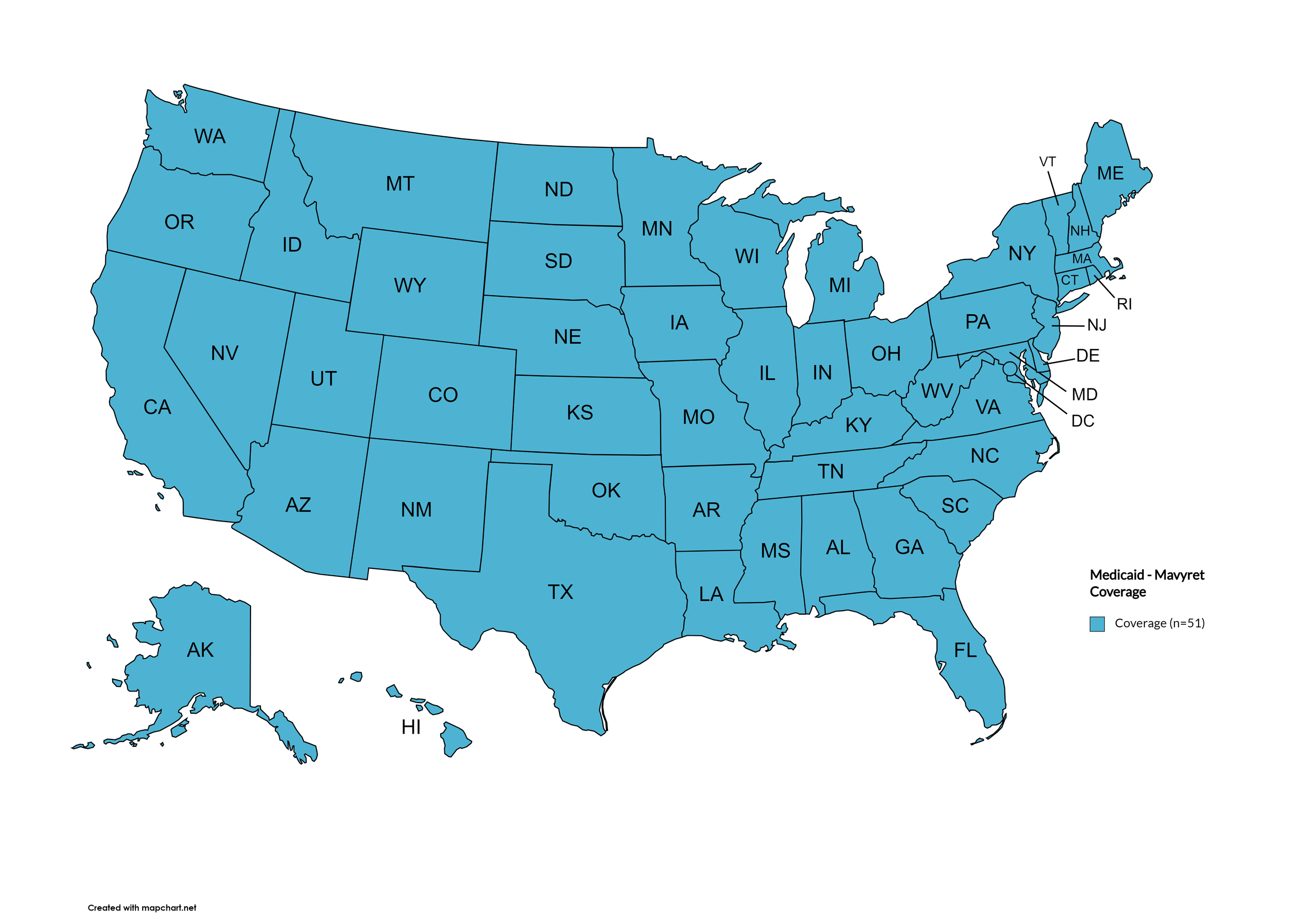
Mavyret
States with Mavyret Coverage: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Figure 17. July 2025 Medicaid Coverage - Mavyret
Map Key: Blue = Mavyret Coverage; Yellow = No Mavyret Coverage/No Information regarding Mavyret Coverage
Pegasys
States with Pegasys Coverage: AK, AZ, AR, CA, CT, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MI, MN, MS, MT, NE, NV, NH, NJ, NM, NC, OH, OK, OR, PA, RI, SD, TN, TX, VT, WA, WV, WI, D.C.
States without Pegasys Coverage: AL, CO, DE, ID, KS, MA, MO, NY, ND, SC, UT, VA, WY
Figure 18. July 2025 Medicaid Coverage - Pegasys
Map Key: Blue = Pegasys Coverage; Yellow = No Pegasys Coverage/No Information regarding Pegasys Coverage
Harvoni (generic)
States with Harvoni (generic) Coverage: AL, AR, CA, CO, DE, GA, HI, IL, IN, KY, LA, MD, MA, MI, MN, MO, MS, MT, NE, NH, ND, OK, PA, RI, SD, TN, TX, UT, VT, WA, WV, WI, D.C.
States without Harvoni (generic) Coverage: AK, AZ, CT, FL, ID, IA, KS, ME, NM, NJ, NV, NC, OK, OH, OR, SC, VA, WY
Figure 19. July 2025 Medicaid Coverage - Harvoni (generic)
Map Key: Blue = Harvoni (generic) Coverage; Yellow = No Harvoni (generic) Coverage/No Information regarding Harvoni (generic) Coverage
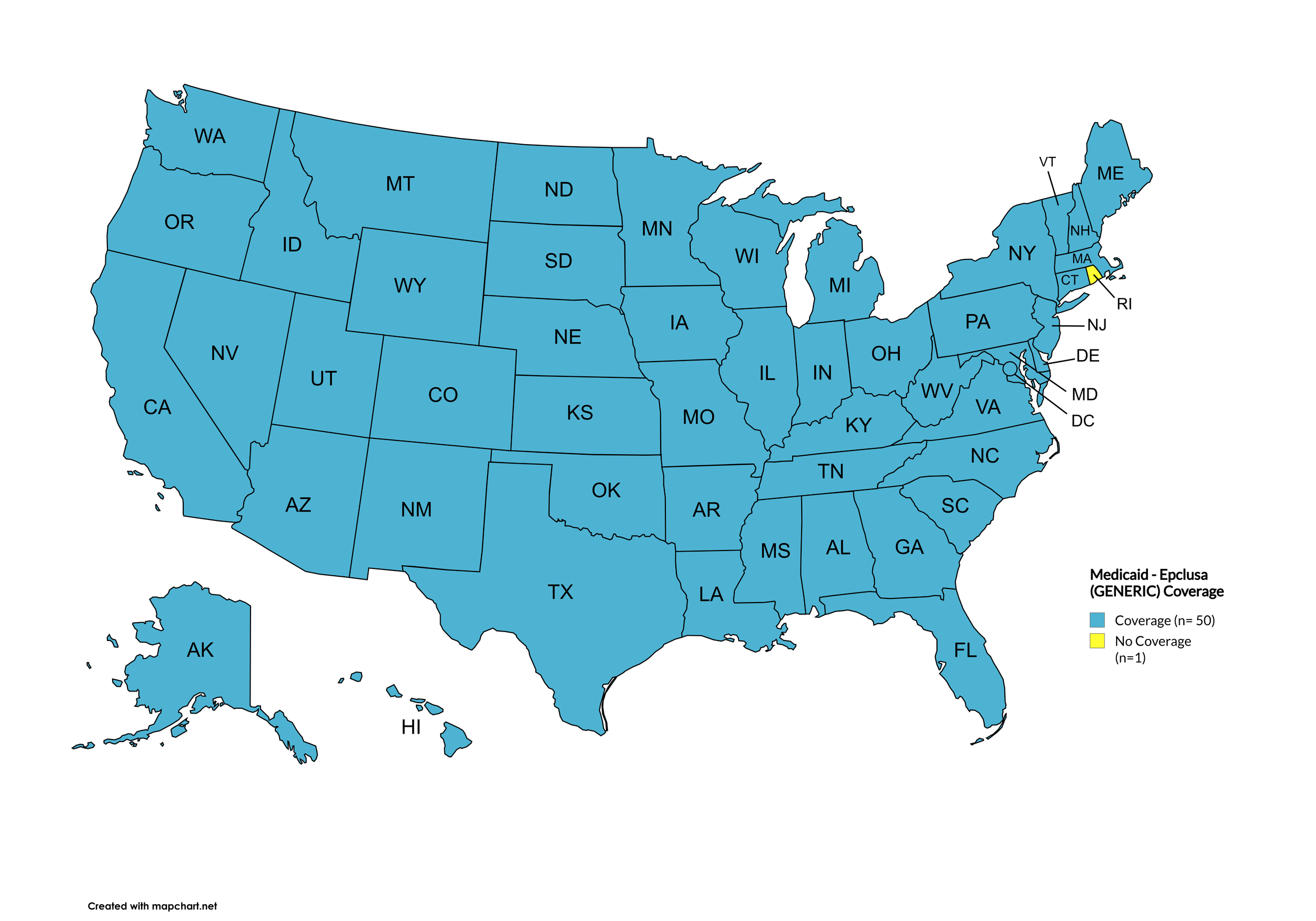
Epclusa (generic)
States with Epclusa (generic) Coverage: AK, AL, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MS, MO, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OK, OH, OR, PA, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Epclusa (generic) Coverage: RI
Figure 20. July 2025 Medicaid Coverage - Epclusa (generic)
Map Key: Blue = Epclusa (generic) Coverage; Yellow = No Epclusa (generic) Coverage/No Information regarding Epclusa (generic) Coverage
July 2025 Notes:
The following states’ Medicaid programs offer multiple coverage plans for their respective Medicaid clients. The plan highlighted in bold typeface represents the most comprehensive plan with the most drugs covered in the respective state:
Hawaii – (1.) Alohacare Advantage Plus; (2.) HMSA; (3.) Kaiser Permanente; (4.) UnitedHealthcare QUEST Integration; (5.) WellCare
New Jersey – (1.) Aetna; (2.) AmeriGroup NJ (Now Wellpoint); (3.) Horizon NJ Health; (4.) UnitedHealthcare of New Jersey (New Jersey Family Care);
New Mexico – (1.) BlueCross BlueShield of New Mexico; (2.) Presbyterian Centennial Care; (3) Western Sky Community Care
Kentucky has a Unified Medicaid Formulary
Louisiana has a Unified Medicaid Formulary
Ohio – Ohio has a Unified Medicaid Formulary that applies to all MCOs
No data has been made available by the Medicaid programs in the U.S. Territories.
Major Policy Changes (April-July 2025):
California no longer covers Vosevi on its Medicaid formulary.
Hawaii Alohacare Advantage Plus plan now covers Pegasys and generic Epclusa. Kaiser Permanente plan now covers generic Harvoni and generic Epclusa.
Louisiana removed prior authorization requirements for generic HCV medications, significantly improving access to sofosbuvir/velpatasvir (generic Epclusa) and ledipasvir/sofosbuvir (generic Harvoni).
Maryland changed Harvoni from preferred to non-preferred status requiring prior authorization, while Mavyret is now preferred with no prior authorization required.
Nevada substantially reduced its HCV formulary by removing Sovaldi, Harvoni, Zepatier, Vosevi, and generic Harvoni, leaving only ribavirin, Pegasys, generic Epclusa, and Mavyret as treatment options.
North Dakota removed prior authorization requirements for Mavyret.
Ohio Medicaid began offering HCV treatment for the first time, with formulary options including ribavirin, Mavyret, Pegasys, and generic Epclusa.
South Carolina added Epclusa to its preferred drug list.
Continuing Policies:
All state Medicaid programs have removed fibrosis restrictions for initial treatment.
There are currently no states that require sobriety as a prerequisite for hepatitis C treatment.
For clarification, if a state requires a complicated prior authorization process or step therapy requiring failure on preferred medications, that is considered 'no coverage'.
Rhode Island remains the only state that does not cover generic Epclusa.
*Medicaid coverage excludes patients from most drug manufacturer patient assistance programs (PAPs)
4. VETERANS PROGRAMS & HCV THERAPIES
The Veteran's Administration (VA) currently offers coverage for all HCV drugs. This is according to the most recent VA National Formulary, dated July 8, 2025 (U.S. Dept. of V.A., 2025a). The VA Treatment Considerations and Choice of Regimen for HCV-Mono-Infected and HIV/HCV Co-Infected Patients, dated March 2021 (U.S. Dept. of V.A., 2021b) lists the following therapies as preferred treatments:
Abbreviations:
- CTP – Child-Turcotte-Pugh (score used to assess severity of cirrhosis)
- IU/mL – International Units Per Milliliter
- PEG-IFN/IFN – Peginterferon/Interferon
- RAS – Resistance-associated substitutions
Genotype 1:
Treatment-naïve without or with cirrhosis (CTP A):
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens:
Zepatier: 1 tablet orally daily for 12 weeks if GT1a without baseline NS5A RAS or GT1b
Harvoni: 1 tablet orally daily
If HCV-noninfected, non-cirrhotic, and HCV RNA baseline <6 million IU/mL: 8 weeks
If cirrhotic, baseline HCV RNA ≥6 million IU/mL, HIV/HCV-co-infected, or African American: 12 weeks
Consider adding ribavirin in CTP A patients
Treatment-naïve with decompensated cirrhosis (CTP B or C):
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Epclusa: 1 tablet orally daily + ribavirin (1000 mg/day - <75kg – or 1,200 mg daily - ≥75kg – orally daily in 2 divided doses with food) for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb).
Treatment-experienced (NS5A- and SOF-naïve [e.g., failed PEG-IFN/RBV ± NS3/4A PI]) without or with cirrhosis (CTP A)
Pangenotypic regimens:
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If NS3/4A PI + PEG-IFN/RBV-experienced: 12 weeks
Vosevi: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks if GT1b, or if failed only PEG-IFN/RBV and GT1a without baseline NS5A RAS
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-naïve and SOF-experienced) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food
If PEG-IFN/RBV + Sovaldi-experienced: 8 weeks if non-cirrhotic or 12 weeks if cirrhotic
If Olysio + Sovaldi-experienced: 12 weeks
Epclusa: 1 tablet orally daily for 12 weeks if GT1b
Vosevi: 1 tablet orally daily with food for 12 weeks if GT1a
Treatment-experienced (prior NS5A-containing regimen) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks if failed only an NS5A inhibitor without NS3/4A PI (e.g., Harvoni)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or C)
Epclusa: 1 tablet orally daily + RBV; start at lower RBV doses as clinically indicated (e.g., baseline Hgb);
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
Genotype 2:
Treatment-naïve or treatment-experienced (PEG-IFN/IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; 12 weeks if CTP A and treatment-experienced or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-naïve or treatment-experienced patients with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 3:
Treatment-naïve without cirrhosis or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks if cirrhotic or in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
If CTP A, test for NS5A RAS
Add ribavirin if Y93H RAS present
Treatment-experienced (PEG-IFN ± RBV or Sovaldi + RBV ± PEG-IFN) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 16 weeks
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
If CTP A, consider adding ribavirin (no supporting data)
Treatment-naïve or treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks
Genotype 4:
Treatment-naïve without or with cirrhosis (CTP A)
Pangenotypic regimens
Mavyret: 3 tablets orally daily with food for 8 weeks; may consider 12 weeks in patients with poor prognostic factors
Epclusa: 1 tablet orally daily for 12 weeks
Non-pangenotypic regimens
Zepatier: 1 tablet orally daily for 12 weeks
Harvoni: 1 tablet orally daily for 12 weeks
Treatment-naïve with decompensated cirrhosis (CTP B or C)
Pangenotypic regimen
Epclusa: 1 tablet orally daily + RBV for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Non-pangenotypic regimen:
Harvoni: 1 tablet orally daily + ribavirin (600 mg/day and increase by 200 mg/day every 2 weeks only as tolerated) for 12 weeks
Treatment-experienced (Sovaldi-experienced and NS5A-naïve) without or with cirrhosis (CTP A)
Mavyret: 3 tablets orally daily with food for 8 weeks if NS3/4A PI-naïve without cirrhosis, and 12 weeks if NS3/4A PI-experienced or CTP A
Epclusa: 1 tablet orally daily + ribavirin for 12 weeks; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
Treatment-experienced (NS5A-experienced) without or with cirrhosis (CTP A)
Vosevi: 1 tablet orally daily with food for 12 weeks
Treatment-experienced with decompensated cirrhosis (CTP B or CTP C)
Epclusa: 1 tablet orally daily + ribavirin; start at lower ribavirin doses as clinically indicated (e.g., baseline Hgb)
If NS5A-naïve: 12 weeks
If NS5A-experienced: 24 weeks; NOT FDA approved for 24 weeks
July 2025 Notes:
Program Stability: The VA HCV treatment program maintained comprehensive coverage throughout the April-July 2025 period with no formulary restrictions, coverage limitations, or policy changes implemented.
Coverage Status: All direct-acting antivirals (DAAs) remain available including:
Sovaldi (sofosbuvir)
Harvoni (ledipasvir/sofosbuvir) and generic formulations
Zepatier (elbasvir/grazoprevir)
Epclusa (sofosbuvir/velpatasvir) and generic formulations
Vosevi (sofosbuvir/velpatasvir/voxilaprevir)
Mavyret (glecaprevir/pibrentasvir)
Ribavirin and Pegasys for combination therapy
Screening and Treatment Success: The VA continues to demonstrate exceptional performance in HCV care with 75% of veterans in care tested for HCV and over 95% of antibody-positive veterans receiving confirmatory testing.
Formulary Updates: The most recent VA National Formulary update occurred July 8, 2025, confirming continued availability of all HCV medications without new restrictions.
Treatment Guidelines: The VA Treatment Considerations and Choice of Regimen guidelines from March 2021 remain current, providing evidence-based treatment recommendations across all HCV genotypes and clinical scenarios.
Comparison to Other Programs: The VA's stable, comprehensive coverage contrasts significantly with the variability and restrictions seen in state ADAP and Medicaid programs, demonstrating the benefits of integrated healthcare system management.
5. PATIENT ASSISTANCE PROGRAMS
The drug manufacturers and various national nonprofit organizations offer a variation of patient assistance programs (PAPs) to assist patients in accessing treatments. They include:
Support Path (Gilead Sciences):
Status: MODIFIED OPERATIONS
Financial Assistance
Provides Co-Pay Coupons for Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), and Vosevi
Co-Pay Coupons cover out-of-pocket costs up to 25% of the catalog price of a 12-week regimen (3 bottles/packages) of Sovaldi, Harvoni, Harvoni (Generic), Epclusa, Epclusa (Generic), or Vosevi
Excludes patients enrolled in Medicare Part D or Medicaid
MAJOR CHANGE: Effective May 5, 2025, transitioned from retail pharmacy to mail order delivery system
MAJOR CHANGE: Discontinued providing free medications for Sovaldi and several HIV medications due to generic availability
Insurance Support
Researches and verifies patient's benefits, and gives information they need about coverage options and policies
Explains Prior Authorization process and works with HCV Specialist's office so they can submit PA forms to a patient's insurance company
May be able to provide assistance with appeals process
Special Exceptions Ended
DISCONTINUED: Special exceptions for Truvada PrEP for individuals assigned female at birth ended July 31, 2025
Website: http://www.mysupportpath.com/
AbbVie Mavyret Co-Pay Savings Card:
Status: ACTIVE
Financial Assistance
Patient may be eligible to pay as little as $5
Excludes patients enrolled in Medicare Part D, Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs
NeedyMeds:
Status: ACTIVE
NeedyMeds Drug Discount Card
Designed to lower cost of prescription medications by up to 80% at participating pharmacies
Price finder tool for the drug discount card
No eligibility requirements
CANNOT be used in combination with government healthcare programs, but CAN be used IN PLACE of program
CANNOT be combined with other offers
Website: http://ow.ly/fEJo309cJ7Z
The Assistance Fund:
Status: WAITLISTED (accepting patients for waitlist)
Requires provider referral
Copay assistance
Eligibility Criteria:
US citizen or permanent resident
Diagnosed with the disease for which you are applying
Prescribed an FDA-approved treatment for the disease
Have prescription coverage for the prescribed treatment
Meet financial eligibility criteria based upon household income and size
Patient Advocate Foundation Co-Pay Relief:
Status: CLOSED
Maximum award of $4,500
Eligibility Requirements:
Patient must be insured, and insurance must cover prescribed medication
Confirmed HCV diagnosis
Reside and receive treatment in the U.S.
Income falls below 400% of FPL with consideration of the Cost of Living Index (COLI) and the number in the household
Patient Access Network (PAN) Foundation:
Status: CLOSED (not currently accepting applications for new or renewal patients)
Co-Pay Assistance with a maximum award of $3,800
PROGRAM ADJUSTMENT: Beginning January 1, 2025, adjusted grant amounts in response to Medicare Part D's new $2,000 out-of-pocket cap, maintaining goal to cover 100% of costs for most patients
Patients may apply for additional assistance during their eligibility period, subject to availability of funding
Eligibility Requirements:
Must be getting treatment for HCV
Have insurance that covers prescribed HCV medication
Medication must be listed on PAN's list of covered medications: https://www.panfoundation.org/index.php/en/patients/medications-covered
Income falls below 500% of FPL
Residing and receiving treatment in the U.S. (citizenship NOT required)
Website: https://www.panfoundation.org/index.php/en/patients/assistance-programs/hepatitis-c
HealthWell Foundation:
Status: OPEN
Co-Pay Assistance with a maximum award of $30,000
Minimum Co-Pay Reimbursement Amount: None
Minimum Premium Reimbursement Amount: None
REOPENED: Fund reopened in April 2025 after previous closure due to insufficient funding
Eligibility Requirements:
Must be being treated for HCV
Have insurance that covers HCV prescribed medication
Income falls below 500% of FPL
Receiving treatment in the U.S.
Website: https://www.healthwellfoundation.org/fund/hepatitis-c/
July 2025 Notes:
Major Program Changes:
Gilead Support Path Program underwent significant restructuring on May 5, 2025:
Transitioned from retail pharmacy distribution to mail order delivery system
Discontinued providing free medications for Sovaldi and several HIV medications due to widespread generic availability
Ended special exceptions for Truvada PrEP for individuals assigned female at birth effective July 31, 2025
Maintained co-pay assistance for hepatitis C medications
PAN Foundation adapted to new Medicare Part D regulations by adjusting grant amounts beginning January 1, 2025, to work within the new $2,000 annual out-of-pocket cap while maintaining comprehensive cost coverage for eligible patients.
Patient Advocate Foundation Co-Pay Relief closed its hepatitis C program to new applications, reducing the overall number of available assistance programs.
The Assistance Fund moved to waitlist status, indicating funding constraints but continued program operation for future applicants.
HealthWell Foundation remains the most significant positive development, maintaining its April 2025 reopening with the highest award amount ($30,000) among active programs.
Impact Assessment: The closure and modification of multiple PAPs reflects the evolving treatment landscape as generic HCV medications become more affordable and accessible. However, these changes may create access gaps for patients who don't qualify for remaining programs or whose insurance doesn't adequately cover newer generic formulations. The HealthWell Foundation's continued operation provides critical support, but increased demand may strain available funding.
Current Active Programs: 4 programs currently accepting new applications (AbbVie, NeedyMeds, HealthWell Foundation, and The Assistance Fund waitlist)
Closed Programs: 2 programs closed to new applications (PAN Foundation, Patient Advocate Foundation)
6. HARM REDUCTION PROGRAMS
Harm Reduction, as it relates to opioid abuse and HCV, are measures designed to serve as preventive or monitoring efforts in combating opioid prescription drug and heroin abuse, and as an effect, helping to prevent the spread of HCV and HIV. The Co-Infection Watch covers the following measures: Syringe Exchange, Expanded Naloxone Access, State Authorized Safe Consumption Sites, Updated Paraphernalia Laws (allowing for possession of substance testing strips), Good Samaritan Laws, Required Prescriber Education. (Editor’s Note: Program descriptions provided herein).
July 2025 Updates:
Syringe Exchange
Syringe Services Programs (SSPs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones. (N.b. – states listed as "at least one SSP…” indicate only that a Syringe Services Program (SSP) exists within the state, regardless of the legality of SSPs under state law).
States with Syringe Exchange: AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, IL, IN, IA, KY, LA, ME, MD, MA, MI, MN, MO, MT, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, D.C.
States without Syringe Exchange: AL, ID, KS, MS, NE, SD, WY
Territories with Syringe Exchange: Puerto Rico, U.S. Virgin Islands
Figure 21. July 2025 Syringe Exchange Coverage
Map Key: Purple = Syringe Exchange(s); Red = No Syringe Exchange(s); Grey = No Information
Expanded Naloxone
Naloxone is a drug used to counteract the effects of opioid overdoses. Expanded Access refers to having statutes or state standing orders in place that allow pharmacies to dispense naloxone without a prescription. This means those in danger of overdose, those who are caregivers for them, or anyone who may come in contact with those in danger of overdose can walk into a pharmacy and obtain naloxone without a prescription. Removing the requirement of a patient-doctor relationship via prescription enhances access.
States with Expanded Naloxone: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Expanded Naloxone: None
Territories with Expanded Naloxone: Unknown
Figure 22. July 2025 Expanded Naloxone Coverage
Map Key: Purple = Expanded Naloxone; Red = Restricted Naloxone; Gray = No Information
State Authorized Safe Consumption Sites
Federal law prohibits the distribution, possession, and consumption of certain controlled substances. Safe Consumption Sites (SCSs) exist to provide injection drug users (or those whose prescriptions require injection) with clean syringes and/or in exchange for used ones, offer wound care supplies, allow for injection drug users to consume drugs, offer infectious disease screening, and other linkage to care opportunities. This section monitors state authorized safe consumption site programs and pilot projects related to safe consumption sites.
States with Safe Consumption Sites: NY, RI
States without Safe Consumption Sites: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NC, ND, OH, OK, OR, PA, SC, SD, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
Territories with Safe Consumption Sites: None
Figure 23. July 2025 State Authorized Safe Consumption Sites
Map Key: Purple = States with sites; Red = States without sites
Updated Paraphernalia Laws
State paraphernalia laws have long prohibited possession of certain drugs use related materials, including harm reduction materials like fentanyl testing strips. Some states have modernized their criminal codes to allow for possession of testing strips and may also have health department programs distributing testing strips.
States with Updated Paraphernalia Laws: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, KS, KY, LA, MD, MA, ME, MI, MN, MO, MS, MT, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, SD, TN, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Updated Paraphernalia Laws: IA
Territories with Updated Paraphernalia Laws: Unknown
Figure 24. July 2025 Updated Paraphernalia laws
Map Key: Red = Without Updated Laws; Purple = With Updated Laws; Gray = No Information
Expanded Good Samaritan Laws
Expanded Good Samaritan Laws are laws that are designed to protect persons seeking emergency services for drug overdoses from drug-related charges or prosecutions, regardless of possession or consumption of illegal, illicit substances, or drug paraphernalia. Good Samaritan laws may or may not provide protection to those currently under parole or probation. Good Samaritan laws listed do NOT prohibit arrest.
States with Expanded Good Samaritan Laws: AZ, CA, CT, DE, Fl, GA, HI, ID, IL, KY, LA, MD, MN, MS, MO, MT, NB, NV NJ, NM, NY, ND, PA, RI, TN
States without Expanded Good Samaritan Laws: AL, AK, AR, CO, IN, IA, KS, ME, MA, MI, NH, NC, OH, OK, OR, SC, SD, TX, UT, VT, VA, WV, WI, WY
Territories with Expanded Good Samaritan Laws: Unknown
Figure 25. July 2025 Good Samaritan Laws Coverage
Map Key: Purple = Good Samaritan Laws; Red = No Good Samaritan Laws; Gray: No Information
Prescriber Education Required
States that require/do not require through legislative action or regulatory or licensing bodies that prescribing physicians undergo special training in addition to or as part of their initial education to become prescribers related to safer controlled substance and/or pain management prescribing and utilization practices.
States with Prescriber Education Required: AL, AK, AZ, AR, CA, CO, CT, DE, FL, GA, HI, ID, IL, IN, IA, KS, KY, LA, ME, MD, MA, MI, MO, MN, MS, NE, NV, NH, NJ, NM, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, UT, VT, VA, WA, WV, WI, WY, D.C.
States without Prescriber Education Required: MT, SD
Territories with Prescriber Education Required: Unknown
Figure 26. July 2025 Prescriber Education Required Coverage
Map Key: Purple = Prescriber Ed Required; Red = No Prescriber Ed Required; Gray = No Information
July 2025 Notes:
Metrics for Mandatory PDMP reporting, Doctor Shopping Laws, Physical Exam, ID Requirements, and Lock-in Pharmacy programs have been permanently deleted from the Watch due to redundancy or outdatedness.
Metric definition for Expanded Naloxone was updated. Medicaid covers naloxone in all states. However, even though naloxone was previously made available OTC federally, some states still required a prescription for it at the pharmacy. Expanded Naloxone designation also addresses issues such as its availability in schools and other public places, the state-by-state variation in who other than pharmacists is allowed to dispense naloxone, and more.
Added metrics for monitoring state paraphernalia laws regarding possession of testing strips and state authorized safe consumption sites, including legislatively authorized pilot projects.
NC, ND, and VT still have controlled substance testing equipment on their drug paraphernalia law statues but provide a carve-out to allow testing strips.
Metric definition for Prescriber Education has been updated to exclude “recommended” and only reflect those states which have laws or licensing board requirements of initial and/or continuing education for prescribers with regard to pain management and/or the prescription of controlled substances.
Some states have general requirements regarding “controlled substances”, some states are explicit with regard to category of controlled substance or type of controlled substance (ie. “opioids”).
This adjustment clarifies that MT and SD are the only states that do not require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
This adjustment clarifies that KS, MO, and ND do require opioid specific and/or pain management specific and/or controlled substances prescribing education by law or licensing institution in either core or continuing education for providers.
The immunity provided under Good Samaritan Laws only applies when there are personal usage amounts of drugs present. It does not apply when there are distribution-level amounts.
In March 2024, Idaho repealed its five-year-old Syringe and Needle Act by passing House Bill 617. The governor signed it into law for it to go into effect July 1, 2024.
Idaho's legalization of fentanyl strips officially went into effect July 1, 2024.
Indiana passed House Bill 1167 to decriminalize fentanyl testing strips, signed by Governor Mike Braun on April 10, 2025, effective July 1, 2025. This removes Indiana from states without updated paraphernalia laws.
Iowa remains the only state that has not updated its paraphernalia laws to allow possession of fentanyl testing strips for harm reduction purposes.
Nebraska's standing order for Naloxone was extended beyond its original August 2024 expiration date.
Rhode Island's first safe consumption site opened in December 2024 at 45 Willard Avenue, next to the Rhode Island Hospital Campus in Providence. The site has served over 500 visitors and prevented 27 overdose deaths in its first six months of operation. The legislature approved a two-year extension of the pilot program in April 2025, extending authorization through March 2026.
Wyoming law does not explicitly outlaw fentanyl testing strips, however they have not been widely distributed.
The DEA's COVID-19-based waiver of in-person exams that was extended through December 31, 2024, has now been extended again through December 31, 2025, as announced in the "Third Temporary Extension of COVID-19 Telemedicine Flexibilities for Prescription of Controlled Medications." The DEA and HHS stated they "continue to carefully consider the input received and are working to promulgate a final set of telemedicine regulations" while extending the current flexibilities.
Additionally, on January 16, 2025, the DEA announced "three new rules to make permanent some temporary telemedicine flexibilities established during the COVID-19 public health emergency while also establishing new patient protections." These proposed rules, currently open for public comment, would create special registration categories for telemedicine prescribing after the temporary extension expires.
State Developments:
California launched a groundbreaking direct-to-consumer naloxone program on April 21, 2025, offering CalRx naloxone at $24 per twin-pack—nearly 50% below standard market prices.
New Hampshire distributed over 58,000 naloxone kits statewide and became the first state to launch comprehensive NaloxBox placement with 192 boxes including 51 in schools.
Maine reported 7,173 overdose reversals using state-supplied naloxone through its tiered distribution initiative.
West Virginia has seen over half of its harm reduction programs close due to restrictive regulations, with only 8 of 19 previously operational SSPs remaining.
Territorial Status:
Puerto Rico and U.S. Virgin Islands maintain syringe services programs.
No territories have authorized safe consumption sites.
Limited data available on territorial naloxone access and Good Samaritan law implementation.
7. LATEST NEWS
Trump Administration Issues Executive Order Targeting Homelessness and Harm Reduction Programs - On July 24, 2025, President Trump signed an executive order titled "Ending Crime and Disorder on America's Streets" that significantly impacts harm reduction and substance abuse treatment programs. The order specifically directs the Secretary of Health and Human Services to ensure that federal grants for substance use disorder prevention and treatment do not fund "harm reduction" or "safe consumption" efforts, which the administration characterizes as facilitating illegal drug use. The order also instructs the Attorney General to review recipients of federal housing assistance that operate safe consumption sites for potential violations of federal law under 21 U.S.C. 856, with the possibility of civil or criminal actions. Additionally, the executive order calls for ending support for "housing first" policies and emphasizes civil commitment for individuals with mental illness and substance use disorders who are living on the streets. The directive requires federal agencies to prioritize grants to states and municipalities that actively enforce prohibitions on open drug use, urban camping, and loitering. This policy shift represents a significant departure from harm reduction approaches that have been supported in many jurisdictions and could impact the availability of services like syringe exchange programs and safe consumption sites that are tracked in the Co-Infection Watch.
Republicans Split on Extending ACA Tax Credits as Premium Increases Loom - Congressional Republicans face internal divisions over extending Affordable Care Act premium tax credits set to expire at the end of 2025, with potential implications for healthcare access among vulnerable populations including those with HIV and hepatitis C. The Congressional Budget Office projects that approximately 5 million Americans will lose insurance coverage by 2034 if the $30+ billion annual funding expires. Trump's pollster Tony Fabrizio warned that Republicans will face a "political penalty" in 2026 midterm elections if the subsidies end, as the policy enjoys broad support even among Trump voters. While some Republicans from competitive districts and states with limited insurance options advocate for extension to prevent premium increases, conservative members like House Freedom Caucus Chair Andy Harris oppose continuation, calling it unaffordable "Covid-era policy." The subsidies currently cap premiums at 8.5% of income for individuals and families above 400% of the federal poverty level. For people living with HIV/HCV co-infection who rely on ACA marketplace plans for coverage of expensive treatments and medications, the expiration could significantly impact access to care, particularly affecting those in states that have not expanded Medicaid. The debate reflects broader Republican tensions over healthcare policy, with some members acknowledging constituents' dependence on ACA coverage while others seek to move beyond the law entirely.
U.S. Quietly Drafts Plan to End Program That Saved Millions From AIDS - The Trump administration is developing plans to phase out PEPFAR (President's Emergency Plan for AIDS Relief), the global HIV treatment program that has saved an estimated 26 million lives over 22 years, according to internal State Department documents obtained by The New York Times. The draft plan calls for "transitioning" countries away from U.S. assistance within 2-8 years, with some nations like Botswana and South Africa facing shutdown within two years, while conflict-affected countries like Haiti and Uganda would have five to eight years. The program would be replaced by "bilateral relationships" focused on disease outbreak detection and creating markets for American drugs rather than providing HIV treatment and prevention services. The documents assume a 42% budget reduction from PEPFAR's current $4.7 billion funding level, despite Congress recently restoring $400 million in planned cuts and expressing continued bipartisan support for the program. Since the Trump administration began reviewing foreign aid, PEPFAR has already been severely disrupted, with partner organizations shutting down after months without funding and prevention programs cut for key populations including sex workers and men who have sex with men. Health experts warn the accelerated timeline is "entirely not feasible," particularly for African countries with high HIV burdens and limited financial resources, with potential reversal of decades of progress in global HIV control efforts.
Global HCV Elimination Goals at Risk as 70% of Target Population Remains Untreated - A comprehensive analysis of hepatitis C treatment patterns across 119 countries from 2014-2023 reveals that only 13 million people have received direct-acting antiviral (DAA) treatment in the past decade, representing just 21% of the initial 62 million with chronic HCV infection and 30% of the WHO's target population for elimination by 2030. The Polaris Observatory study found that Egypt, Pakistan, the United States, and European Union accounted for 67% of all HCV treatments, highlighting significant regional disparities in access to care. Treatment peaked in high-income countries in 2016 but declined 8% before COVID-19 caused an additional 29% drop in 2020, while lower middle-income countries experienced a 22% decline pre-pandemic followed by a 44% COVID-related decrease. The research identified a strong relationship between DAA pricing and treatment access, particularly in low- and middle-income countries where cost remains a major barrier. With approximately 70% of the target population still untreated and only six years remaining until the WHO's 2030 elimination goal, experts emphasize the urgent need for accelerated national responses, expanded screening programs, and improved linkage to care to achieve global HCV elimination targets.
New Study Finds Evidence of Hepatitis C Virus in Cells Lining Human Brain - Johns Hopkins Medicine researchers discovered hepatitis C virus in the choroid plexus—cells lining the brain's fluid-filled cavities—of individuals with schizophrenia and bipolar disorder, providing the first direct evidence of viral presence in brain tissue associated with psychiatric conditions. The study analyzed postmortem brain samples and electronic health records from 285 million patients, finding that HCV was present only in the brain lining of people with schizophrenia or bipolar disorder, but not in controls or those with major depression. Electronic health records revealed HCV rates of 3.6% in schizophrenia patients and 3.9% in those with bipolar disorder—nearly double the rate in major depression (1.8%) and sevenfold higher than controls (0.5%). Importantly, the virus altered gene expression in the hippocampus despite not being present in that brain region, suggesting a mechanism by which brain lining infections could affect behavior and cognition. The findings challenge assumptions that higher HCV rates in psychiatric patients result solely from risky behaviors like drug use, instead suggesting viral infection may contribute to disease causation. Since HCV is treatable with antiviral medications, researchers propose screening psychiatric patients for the virus could lead to new treatment approaches that address both the infection and associated symptoms.
Female Sex, Recent Pregnancy Linked to Poor HCV Treatment Rates in Opioid Use Disorder - A retrospective study of 22,347 episodes involving people with hepatitis C entering opioid use disorder treatment found that female sex and recent pregnancy are independent risk factors for reduced direct-acting antiviral prescription rates. The analysis revealed significant treatment disparities: 40.6% of men received DAAs within one year compared to 35.7% of women without recent pregnancy and only 31.8% of women with recent pregnancy. Using data from commercial and Medicaid databases, researchers identified that men and women without recent pregnancy had significantly higher HCV treatment rates compared to women with recent pregnancy (adjusted hazard ratios of 1.18 and 1.09, respectively). The findings highlight a critical gap in postpartum HCV care, with previous research showing only 6% of women with Medicaid, OUD, and HCV infection received DAAs within six months of delivery. Given that HCV can be transmitted from mother to baby and that people with HCV often remain asymptomatic for years, researchers suggest pregnancy could represent a missed opportunity for treatment intervention. The study underscores the need for improved care delivery strategies specifically targeting pregnant and postpartum individuals with co-occurring HCV and opioid use disorders to address these documented treatment disparities.
Pioneer HIV researcher Robert Gallo: Cuts to medical research funding leave us vulnerable to viral threats - HIV co-discoverer Robert Gallo warns that significant cutbacks in medical science funding, particularly for global health research and surveillance, are leaving the world vulnerable to emerging viral threats like H5N1 avian influenza. Drawing parallels to the early AIDS crisis when infectious disease departments were being closed due to false confidence that such diseases were "problems of the past," Gallo emphasizes that sustained investment in research infrastructure enabled the rapid response that led to HIV's identification, blood testing, and life-saving treatments. He highlights concerning trends with H5N1, which has evolved from primarily affecting birds to infecting mammals, including over 1,000 U.S. dairy herds and causing more than 70 human infections since 2022, with over 168 million poultry culled domestically. While the virus hasn't yet mutated for human-to-human transmission, Gallo stresses the urgent need for enhanced surveillance, particularly at human-animal interfaces, and notes that global genomic monitoring remains inadequate—with only 0.5% of COVID-19 cases sequenced compared to recommended levels for real-time variant detection. He advocates for strengthening public-private partnerships, cross-border collaboration, and science-driven solutions, warning that innovation requires years of basic research investment rather than reactive funding after outbreaks begin, emphasizing that "if you invest more, progress comes sooner; if you invest less, it comes later."
Is HIV integration a response or a death sentence? Communities demand answers - At the 13th International AIDS Society Conference in Kigali, Rwanda, heated debates emerged over HIV service "integration"—moving HIV care from specialized clinics into general healthcare facilities—as countries respond to US funding cuts. While integration has been a WHO goal for decades, advocates like Yvette Raphael from Advocacy for Prevention of HIV in Africa warned that "without decriminalisation, accountability and the funding needed, integration will be a death sentence for us." Countries like Uganda and Zimbabwe are rapidly phasing out standalone HIV clinics and transferring services to primary healthcare, but key populations—including sex workers, men who have sex with men, and people who use drugs—face significant barriers in accessing care through general facilities due to criminalization and stigma. Advocates argue that current integration efforts focus only on drug dispensing rather than holistic care, and worry that rushed implementation prioritizes efficiency over quality and equity. Successful models from Southeast Asia show promise when governments provide targeted support: Vietnam achieved 95% HIV social health insurance coverage after waiving premiums for vulnerable groups, while Thailand's "social contracting" model reimburses community organizations for key population services. Conference participants emphasized that communities must lead integration discussions and that evidence-based approaches are essential to ensure integration doesn't drive vulnerable populations underground or reverse decades of progress in reaching marginalized groups with HIV services.
Structural bioinformatics approaches for predicting novel drug targets in hepatitis C virus proteins: a comprehensive analysis - Researchers used computational structural biology methods to identify new potential drug targets in hepatitis C virus proteins, focusing on the NS3 protease, NS5B polymerase, core protein, and NS5A. The study employed homology modeling, molecular docking, and molecular dynamics simulations to analyze HCV protein structures and predict druggable binding sites. Key findings included detailed characterization of binding pockets and interaction patterns that could guide rational drug design for anti-HCV therapeutics. While the computational predictions showed promising results with favorable binding interactions for potential small-molecule inhibitors, the authors emphasize that experimental validation through binding assays and antiviral efficacy testing is needed to confirm the therapeutic potential of these targets. The research contributes to ongoing efforts to develop novel treatments beyond current direct-acting antivirals, particularly important given challenges with drug resistance and accessibility in HCV treatment.